- Home >
- Test papers >
MCQ on stereochemistry: Page-8
What is the configuration of the below molecule
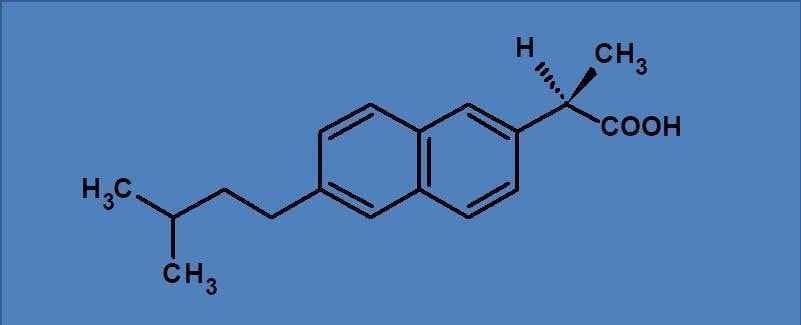
(A) 1R
(B) 1S
(C) 2R
(D) 2S
For most of the chiral drugs, which enantiomer is more active?
(B) R isomer
(B) S isomer
(C) It can’t be generalised
(D) Always their racemic mixture
A stereoselective reaction produces
(A) Only one stereoisomers
(B) More percent of one stereoisomer
(C) More recemic mixture
(D) A meso product
The reactants in stereospecific reaction are
(A) Stereoisomers
(B) Constitutional isomers
(C) May not be stereoactive
(D) None
Optical rotation of a stereoactive compound depends on

(A) R
(B) S
(C) P,Q
(D) R,S