Chemistry of phenothiazine antipsychotics
by egpat 06 Aug 2019
Phenothiazines are one class of old generation antipsychotics with lot of interest focussed on their chemistry. Many of the drugs in this category are structurally related and even they show a significant relation between their antagonsim at dopamine receptors and their structure.
Chemical features
What is the common structure of these phenothiazines? Illustrative from name of their chemical class, they contain two heteroatoms. One is “thia”, being sulphur and another is “aza” being nitrogen. These two heteroatoms are included in a six membered ring system indicated by suffix “ine”. Finally this heterocyclic ring is fused with phenyl rings to form phenothiazines.
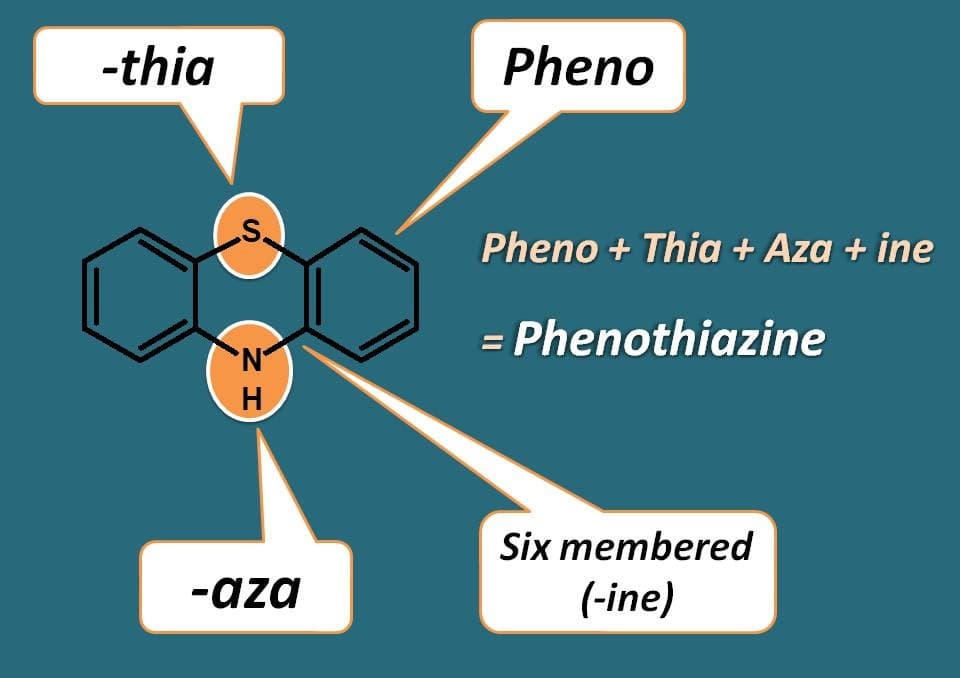
That’s great! Now they have three rings with central ring having heteroatoms. Where they are and what are their positions? For that, we have to give numbering to the ring system. As sulphur should be given least possible numbering than nitrogen, sulphur gets 5th position while nitrogen gets 10th position.

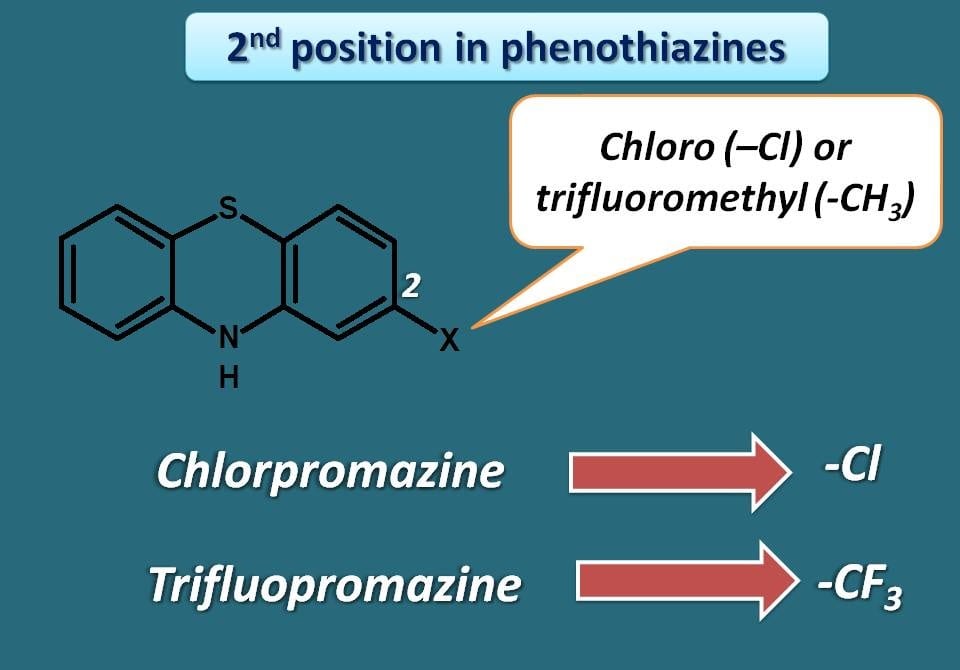
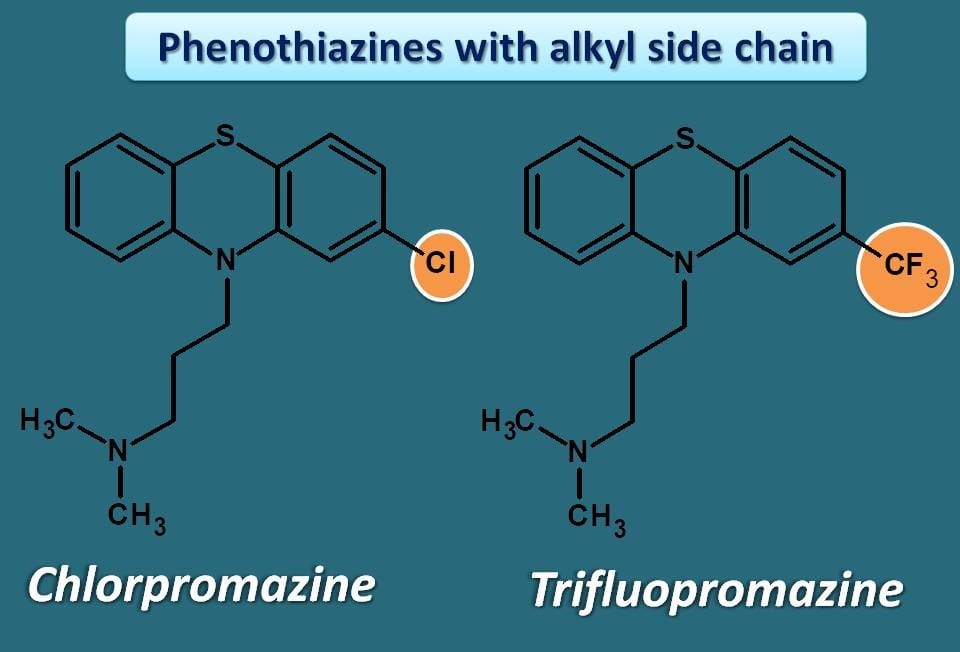
Many of phenothiazines require an electron withdrawing group at 2nd position to produce their antipsychotic action. You can find groups like chloro (–Cl) or trifluoromethyl (–CF3) at 2nd position in many of the phenothiazines. You can also notice these groups represented in the names of few phenothiazines. For example, chlorpromazine has chloro group and trifluopromazine has –CF3 group.
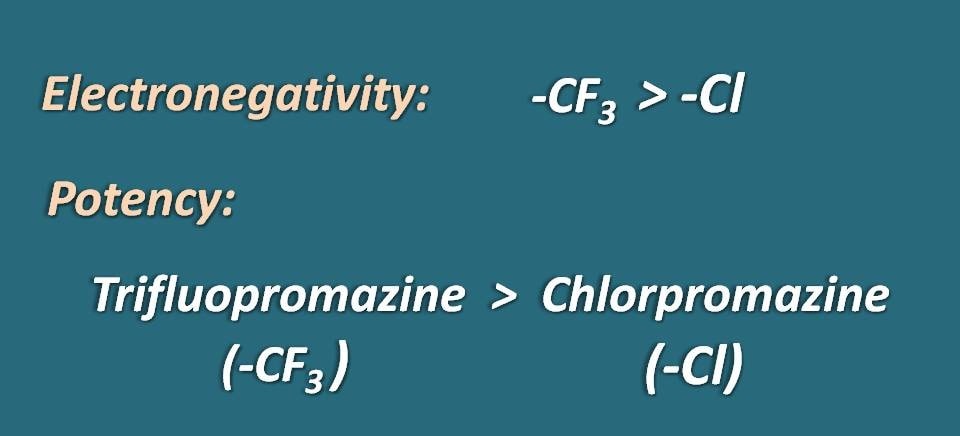
It was also noticed that the potency of phenothiazine was increased with the electronegativity of these groups. Since –CF3 is more electronegative than –Cl group drugs that have the former group at 2nd position are more potent. That’s why trifluopromazine is more potent than chlorpromazine both of the drugs differ only at the 2nd position.
But just wait! The group at 2nd position is not playing the entire role in influencing potency and activity. One of the important and essential chemical features of phenothiazines is their side chain at 10th position. Based on the nature of this side chain, we can have different chemical classes of phenothiazines with variable activity and potency.
Chemical classification
Phenothiazines include several drugs that differ with the side chain they have. Based on the nature of this side chain they can be categorised into three classes.
- Alkyl side chain
- Piperidinyl side chain
- Piperazinyl side chain
Phenothiazines with alkyl side chain
Chlorpromazine, trifluopromazine are two important drugs in this category. You can observe the drug suffix “-promazine” where pro indicates propyl side chain.
Phenothiazines with piperidinyl side chain
Only one drug is currently available in this group. That is Thioridazine.
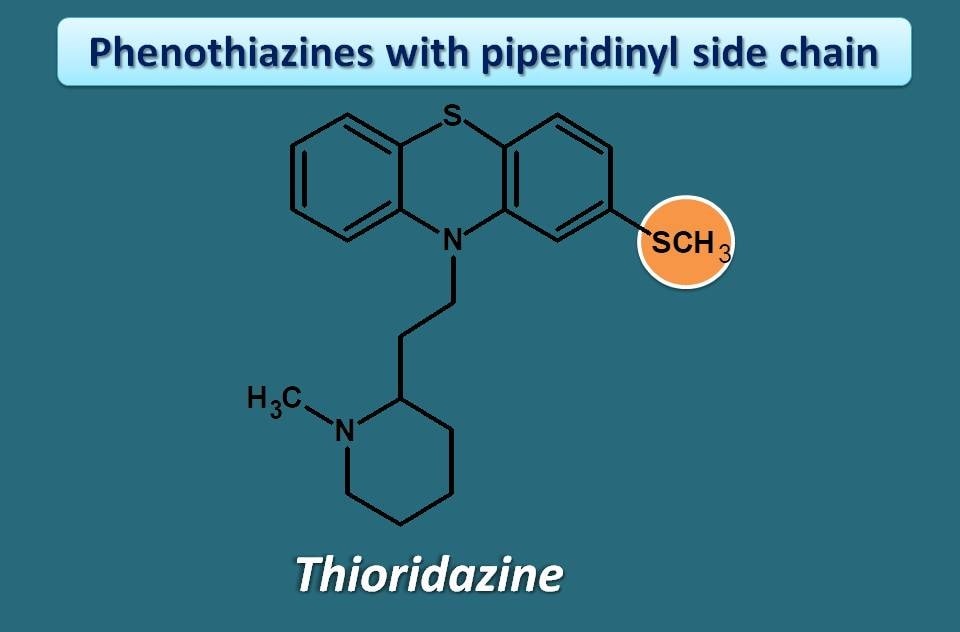
Among the phenothiazines, this is the drug that has less risk of extrapyramidal side effect (EPS). This is not related with its structure but it was thought due to its high antichoinergic action. Generally if an antipsychotic blocks cholinergic receptors, it may have less chance to produce motor disturbances like EPS.
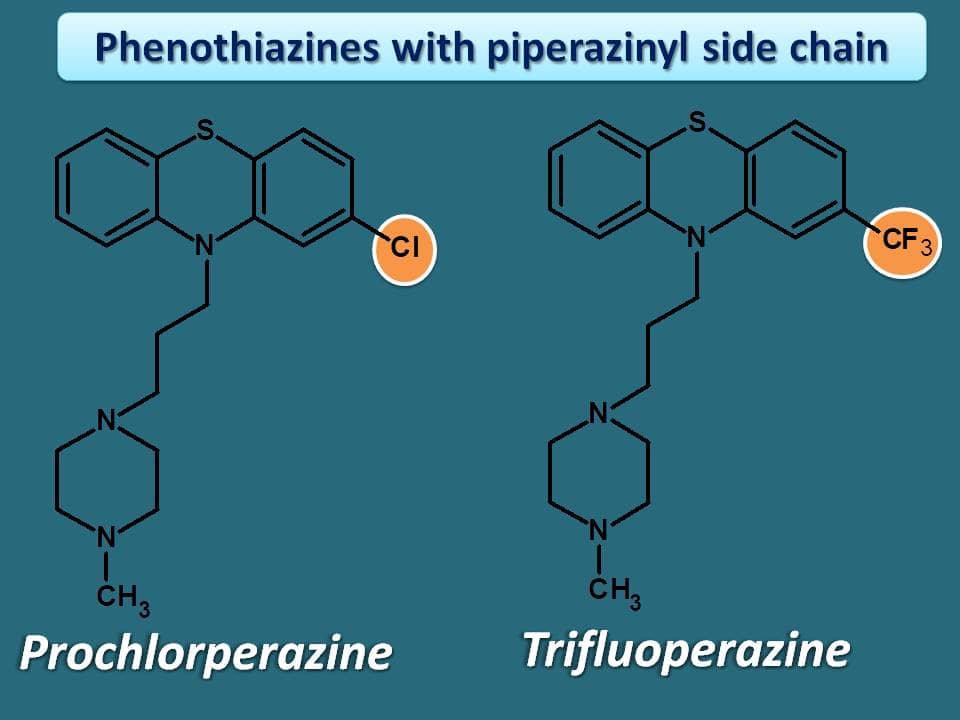
Phenothiazines with piperazinyl side chain
This is the most potent group in this category. Earlier drugs in the class include prochlorperazine and trifluoperazine. Again these two drugs have –Cl and –CF3 groups at 2nd position respectively.
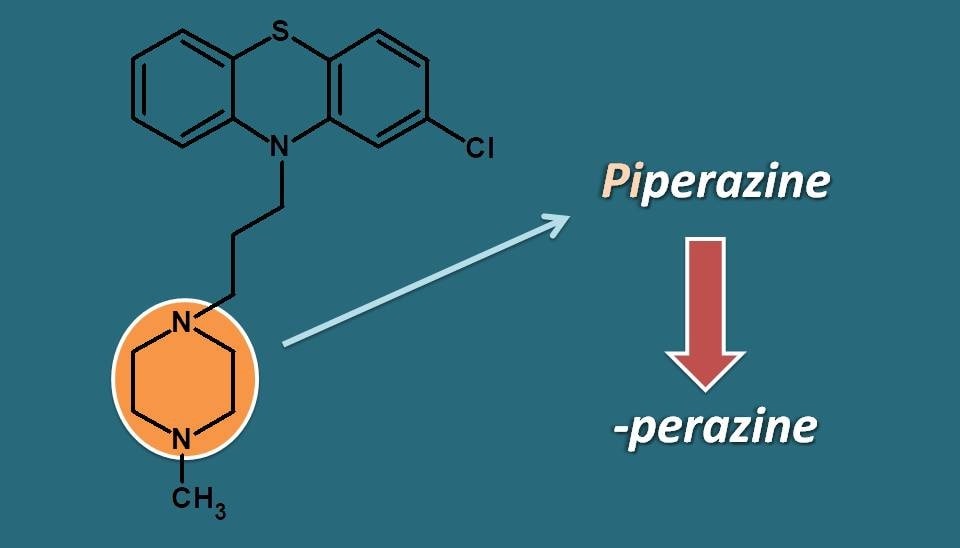
Phenothiazines can have drugs with multiple suffixes . One group of drugs already seen earlier, have the suffix “promazine”. Now, you can notice that drugs in this group have N-methyl piperazine ring attached to alkyl side chain on phenothiazine ring. Here again the suffix “-perazine” indicates that these drugs have “piperazine” as side chain.
Other drugs which are related to above two drugs in this class are perphenazine and fluphenazine with simple modification of side chain. These two drugs have hydroxyl ethyl group on nitrogen of piperazine ring.
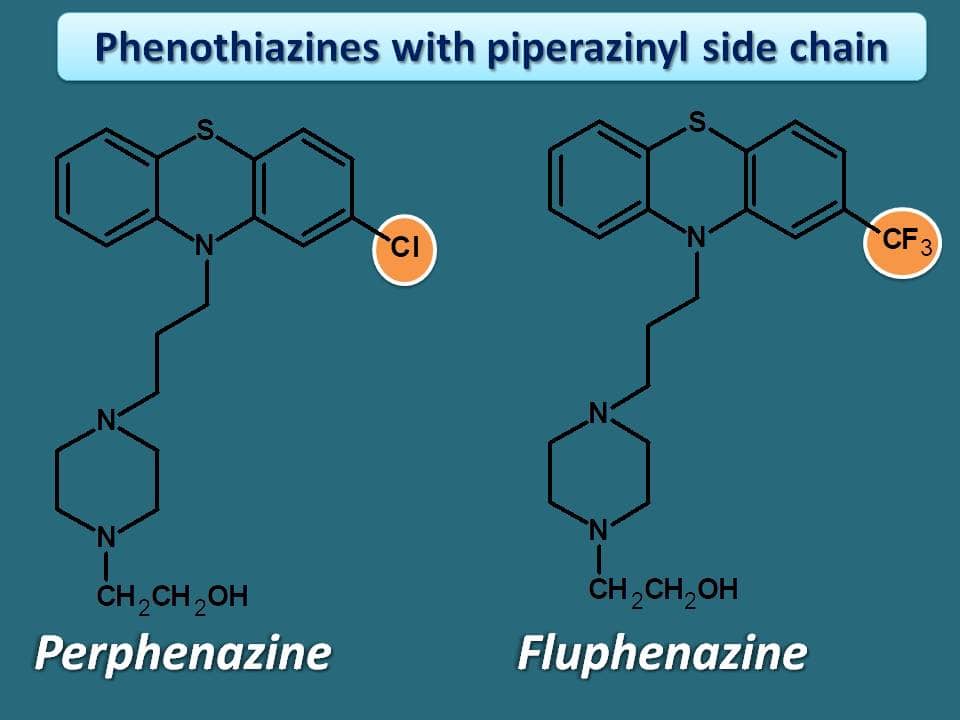
Fluphenazine was observed as the more potent drug in this category.
Effective size of side chain
You have seen many of the phenothiazines are closely related with a slight difference at side chain.
Can you find any common feature again on these side chains?
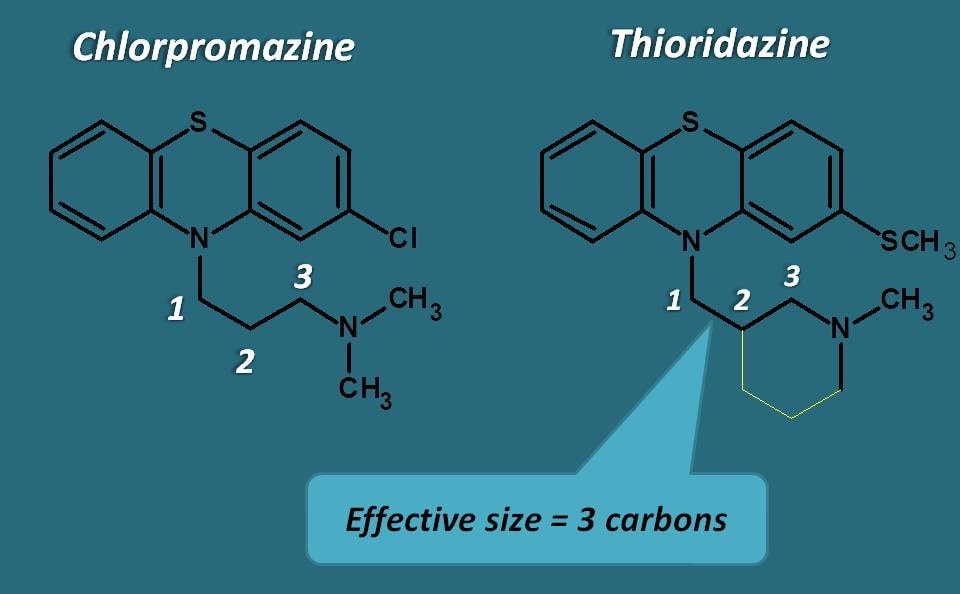
Definitely, one thing is in common among them. That is length of the alkyl side chain. Except, thioridazine all other drugs have aliphatic chain with three carbons. It was also found that, the activity will be lost if the chain length is either increased or decreased.
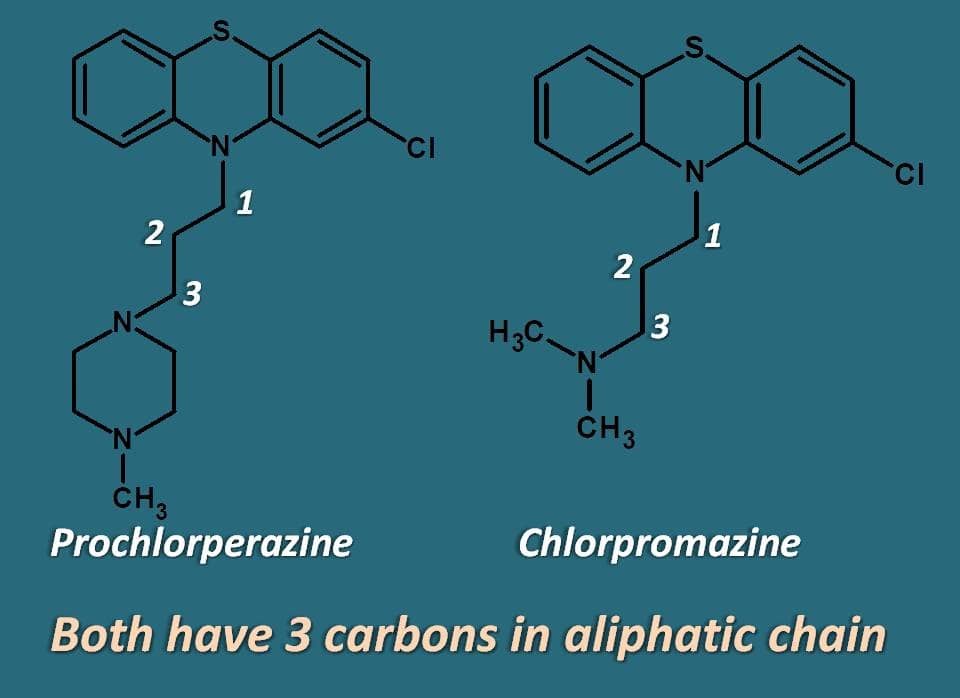
Why three carbons in side chain?
It’s all due to action of phenothaizines on dopamine receptors. In order to bind to dopamine receptors, these drugs should form a hydrogen bond between electronegative atom at 2nd position of phenothiazine ring and nitrogen of alkyl side chain. This is possible with carbon chain of three carbons only which brings nitrogen nearer to electronegative atom to form hydrogen bond.
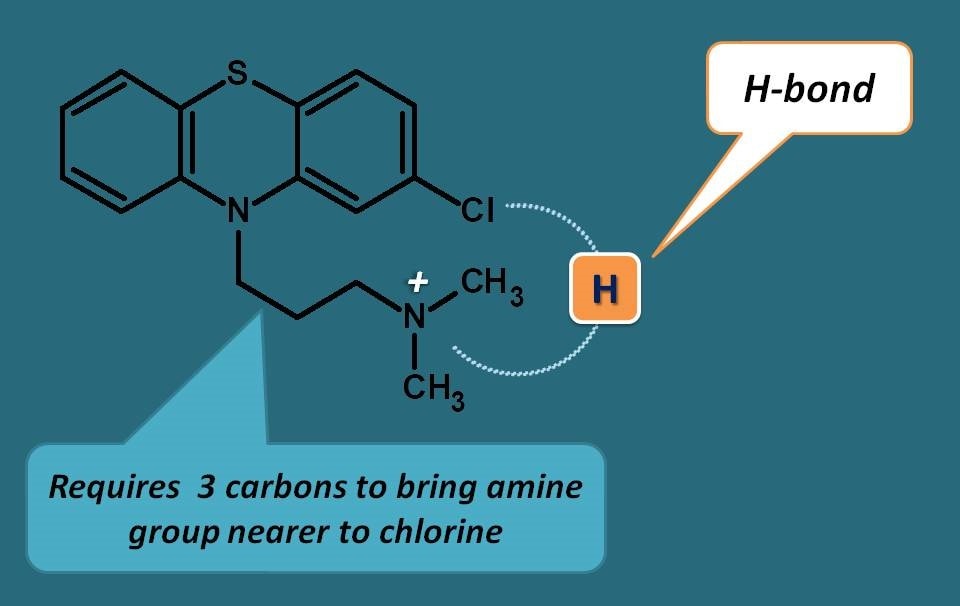
Then what about thioridazine? It has no alkyl chain, then how it can bind to dopamine receptors?
This is a point to consider. But we have reason for this. That is “Effective size”.
Yes, even it has no alkyl chain , the piperidinyl ring has an effective size of three carbons of aliphatic chain bringing the nitrogen into the vicinity of electronegative atom forming H-bond.