- Home >
- Test papers >
MCQ on UV-Visible spectroscopy: Page-4
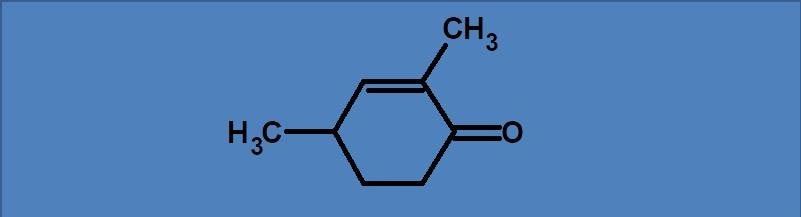
(A) 202 nm
(B) 215 nm
(C) 253 nm
(D) 230 nm
Enone is a α, β-unsaturated carbonyl compound acts a chromophore just like a diene. In a cyclic form with 6 member ring, it shows base value at 215 nm. Even acyclic enone also has the same base value as the cyclic six member enone. 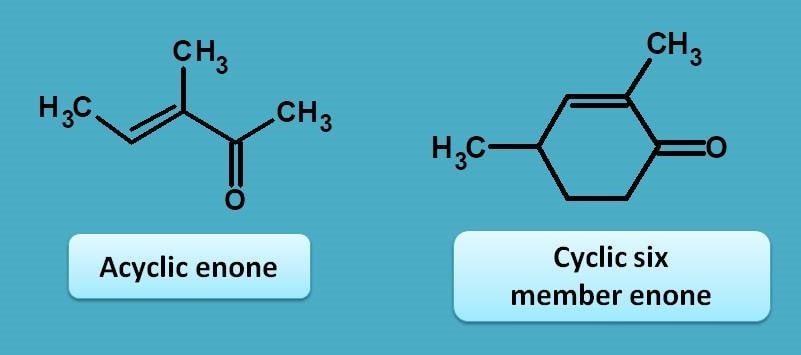

(A) P,Q
(B) P,R
(C) P,Q,R
(D) P,Q,R,S
Since it has both pi and n electrons, it can undergo pi to pi* and n to pi* transitions.
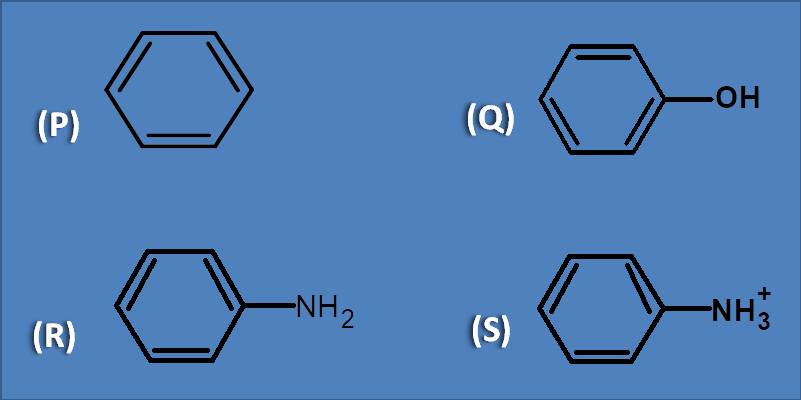
(A) Benzene
(B) Phenol
(C) Aniline
(D) Anilinium ion
As we know that auxochromes increase absorption, here we have to see which compound has strong auxochrome. Again the capacity of an auxochrome to increase absorption depends on the extension of conjugation by lone pair of electrons. Benzene has no auxochrome while anilinium ion has lost lone pair of electrons hence not acts as auxochrome. Now among phenol and aniline, the later has amine group which can show more auxochromic effect as nitrogen has low electronegativity than oxygen.
(A) 18
(B) 12
(C) 10
(D) 5
(A) Wood ward fieser
(B) Nielsen rules
(C) Fieser-kuhn rules
(D) All of the above