- Home >
- Test papers >
MCQ on complexometric titrations: Page-4
Calculate the solubility of the following salt
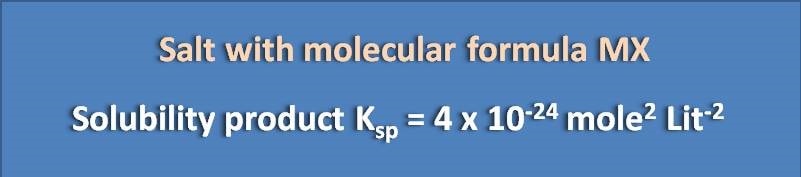
(A) 1 x 10 -8 M
(B) 2 x 10-12 M
(C) 4 x 10 -8 M
(D) 1 x 10 -12 M
To the salt solution in the above question, another salt of 0.01 M NaX was added. Now the solubility of salt MX will be
(A) 1 x 10 -5 M
(B) 2 x 10 -22 M
(C) 4 x 10 -8 M
(D) 2 x 10-11 M
Replacement titration is used for
(A) Calcium
(B) Magnesium
(C) Zinc
(D) All of the above
All of the following acts as masking agents, except
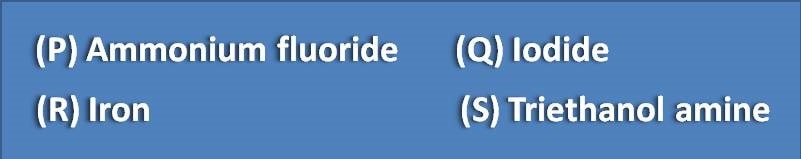
(A) R
(B) Q
(C) P,Q
(D) R,S
In alkalimetric titrations, the titrant is
(A) Iodine
(B) EDTA
(C) NaOH
(D) Magnesium