- Home >
- Test papers >
MCQ on poentiometry: Page-4
Calculate the emf of following concentration cell 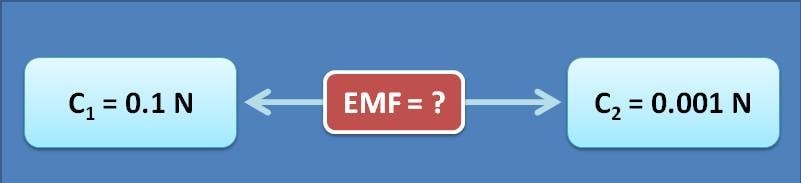

(A) 0.012
(B) 0.023
(C) 0.059
(D) 0.108
In Daniel cell Zn/ZnSO 4 acts as
(A) Cathode
(B) Anode
(C) Both
(D) Reference electrode
The standard electrode potential for a half cell is + 4.5 V. The metal is
(A) More easily oxidized than hydrogen
(B) Less easily oxidized than hydrogen
(C) Cannot be predicted
(D) It depends on other half cell
The reference electrode that is thermally more stable is
(A) Calomel electrode
(B) Silver-silver chloride electrode
(C) Both are equally stable
(D) Both are unstable
Voltammetry belongs to the group of electrochemical analysis of
(A) Steady-state methods
(B) Transient methods
(C) Controlled potential methods
(D) Charge transfer by migration