Why stearic acid has more melting point than oleic acid?
by egpat Posted on 08-07-2017
Both stearic acid and oleic acid are C18 fatty acids but differ in their unsaturation. Stearic is a saturated C18 fatty acid whereas oleic acid is an unsaturated C18 fatty acid.
Saturated fatty acids will show different physical properties compared with unsaturated fatty acids of similar chain length. One of the important property is melting point.
Two factors mainly influence melting point of fatty acids. One is molecular weight and another is unsaturation.
1. Molecular weight
As common with all hydrocarbons, melting point increases with molecular weight.
For example, stearic has a melting point of 69oC whereas palmitic acid has 63oC.
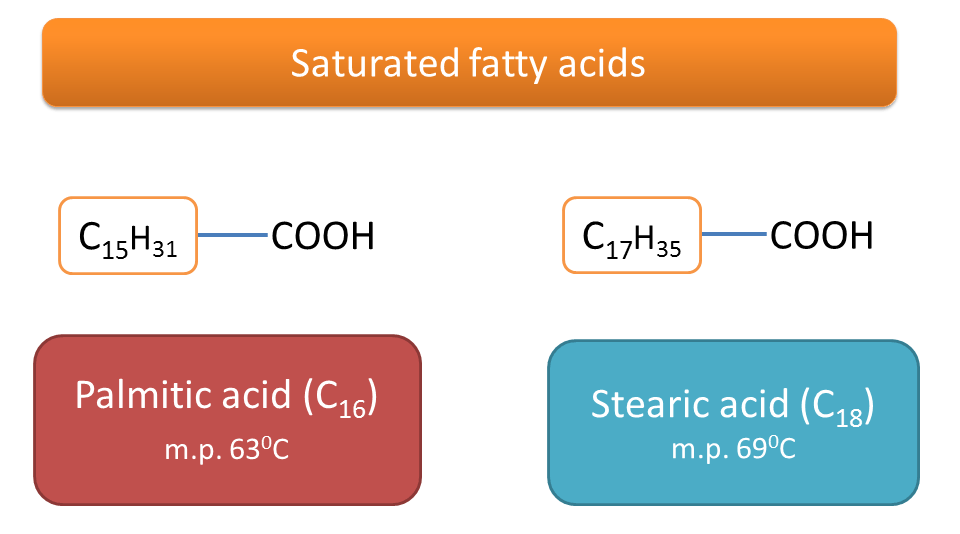
Stearic acid is a C18 fatty acid and palmitic acid is C16 fatty acid. Both are saturated fatty acids but stearic acid (C18) has more molecular weight than palmitic acid (C16) hence more melting point.
Molecular weight increases with number of carbons and hence chain length of the fatty acid. Therefore C18 fatty acids will have more molecular weight than C16 fatty acids.
As the chain length increases, the Van der Waals forces between fatty acid molecules increases thereby intermolecular forces increase. This results in an increase in the melting point.
2. Unsaturation
Now let’s consider second factor, unsaturation.
Again common with all hydrocarbons, melting point decreases with unsaturation.
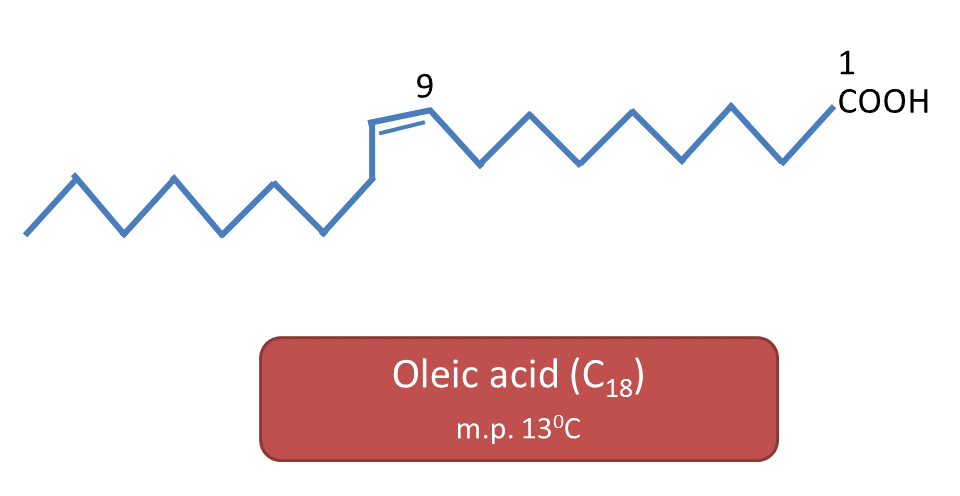
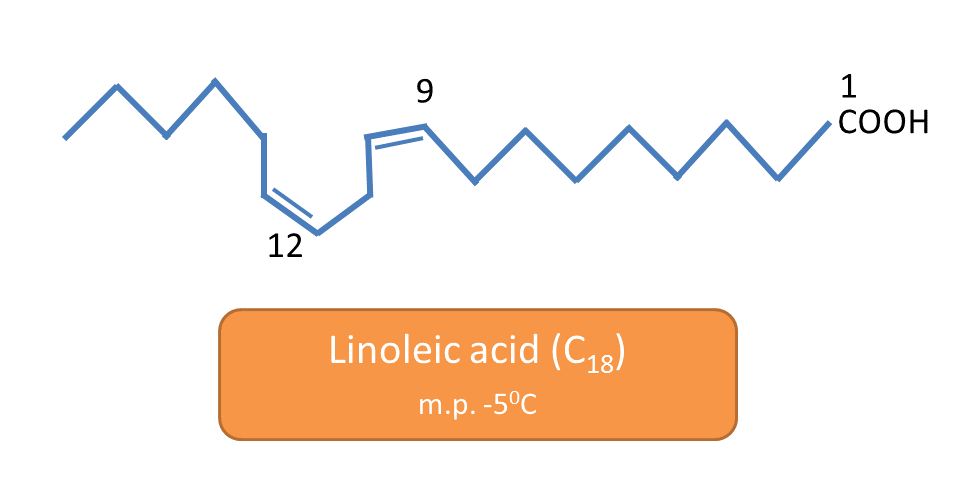
For example, oleic acid, linoleic acid and linolenic acid all are unsaturated C18 fatty acids. Oleic acid melts at 13oC, linoleic acid at -5oC and linolenic acid at -11oC.
Here the number of double bonds play important role.
Oleic acid has one double bond,
linoleic acid has two
and linolenic acid has three double bonds.
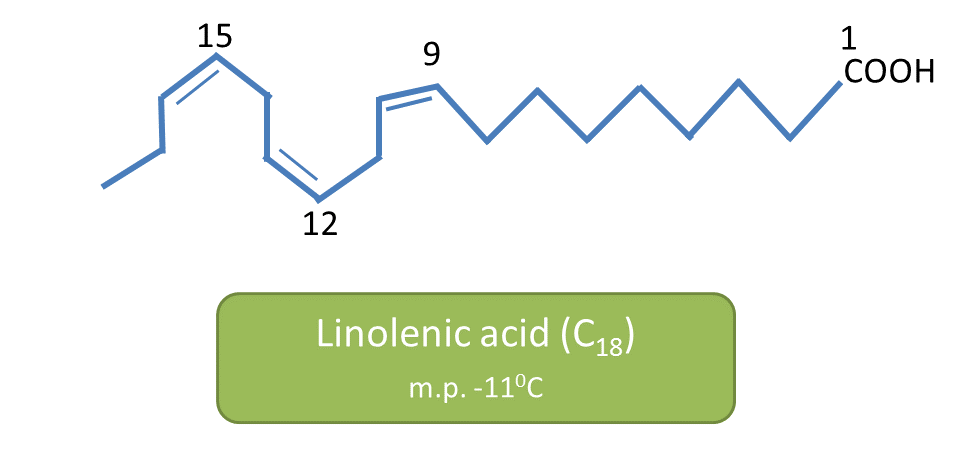
As the number of double bonds (unsaturation) increases, the molecule tends to lie in non-linear fashion and attains more spherical structure. Due to spherical, structure poly unsaturated fatty acids show less contact with other molecules hence less intermolecular interaction.
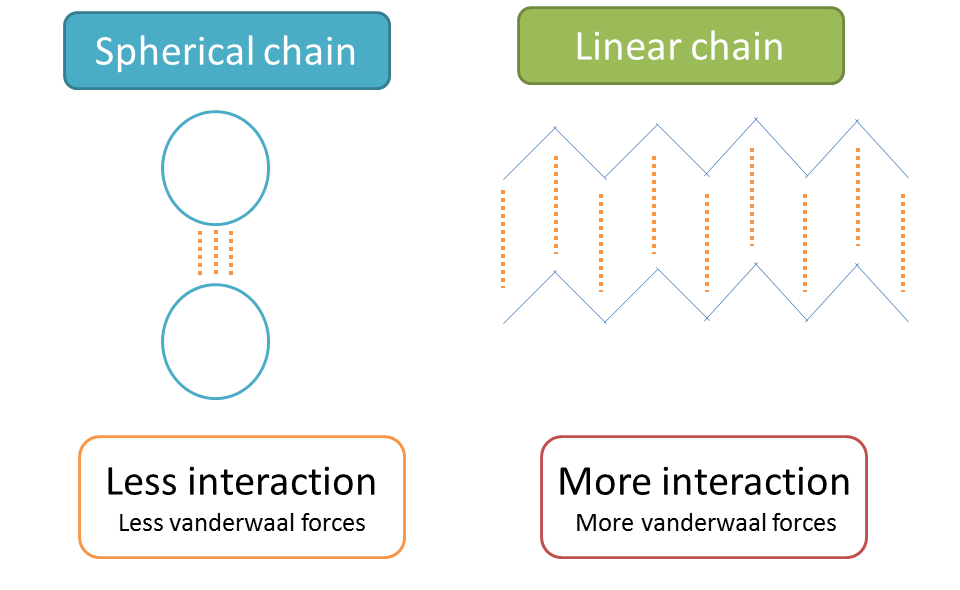
As the intermolecular interaction decreases, Van der Waals forces between molecules decrease and melting point decreases.