What are the annulenes?
by egpat Posted on 22,Mar 2019
Let’s draw a cyclic ring and put double bonds alternatively. You can start with ring sizes 3,4, 5...so on and you can increase ring size as you wish. Interestingly you can find a common thing in all these rings. All these balloons are beautifully made with carbons joining their hands with systematically organized double bonds. Yes, these are the annulenes which are monocyclic, containing conjugated double bonds and prefixed with their ring size.
Molecular formula of annulenes
As annulenes are monocyclic rings with conjugated double bonds, the molecular formula linearly increases with ring size with a little difference with odd number or even number.
For instance, if we take a ring with odd number size such as 3, the ring contains 3 carbons, one double bond and 4 hydrogens.
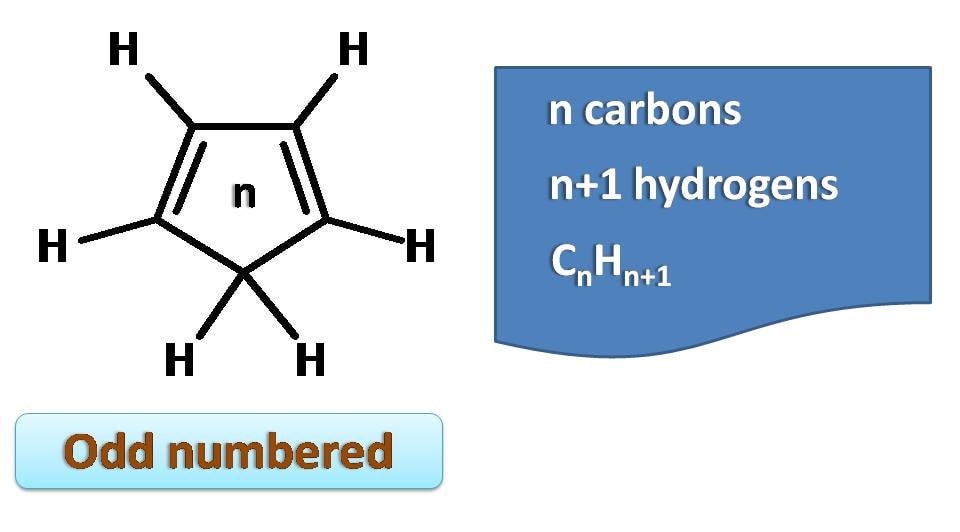
So the molecular formula is C3H4.
We can generalise this as CnHn+1.
On the other hand, even numbered annulenes contain half of the double bonds and equal number of hydrogens to their ring sizes.
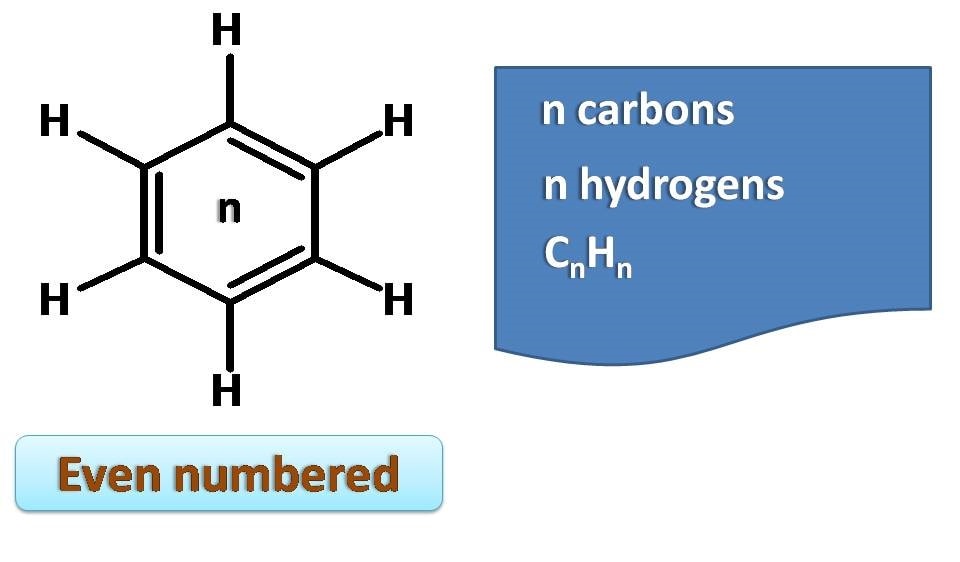
So a 4 membered ring will have two double bonds and the molecular formula is C4H4.
Odd numbered annulenes
Few of the annulenes with odd numbered ring size are cyclopropene, cyclopentadiene and so on.
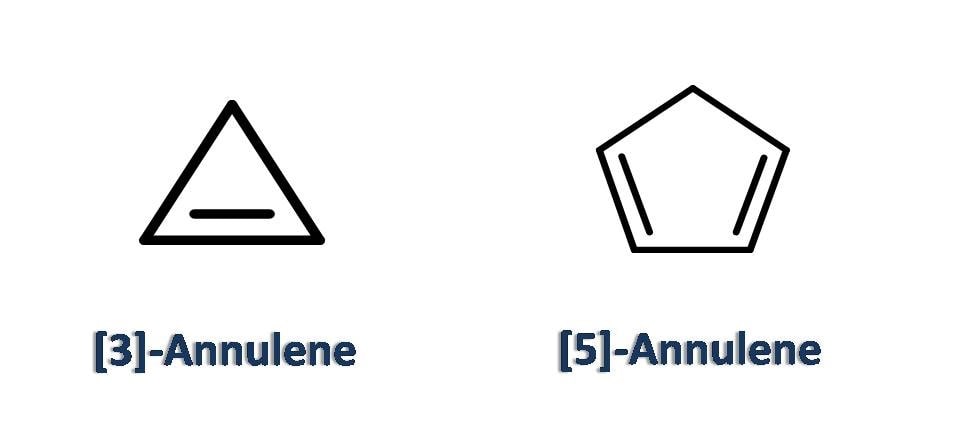
These annulenes are not that much stable as they exhibit ring strain and they can easily relieve ring strain by undergoing ring opening reactions and addition reactions.
Even numbered annulenes
These annulenes are of somewhat interesting due to the following properties.
- All the carbons are sp2 hybridized.
- All carbons have a p orbital which forms pi cloud above and below the ring.
- Many of the rings are flat structures with no ring strain.
- Majority of the rings are aromatic and hence highly stable.
Let’s take [4]-annulene.
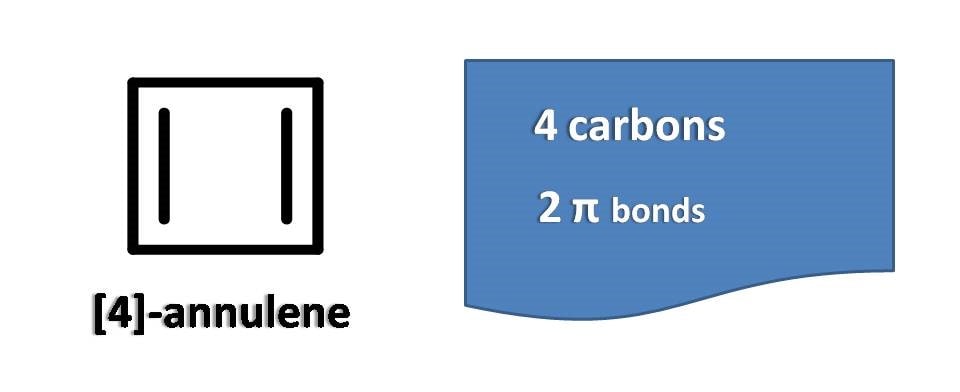
It is a four membered ring with two double bonds and commonly known as cyclobutadiene. Of course, it is not aromatic as it contains only 4 pi electrons which doesn’t match with condition of aromaticity.
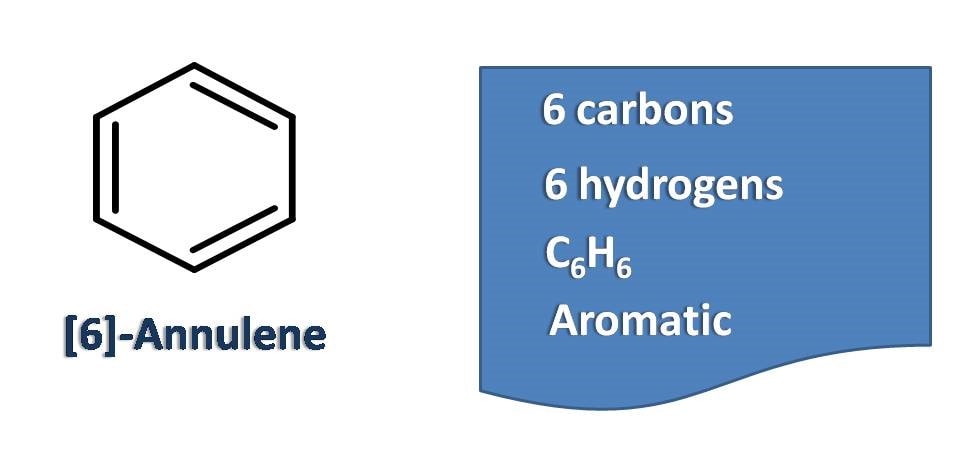
Well known compound benzene is [6]-annulene which is aromatic and highly stable.
Similarly, [8]-annulene and [12]-annulene are not aromatic as they don’t obey Huckel’s rule.

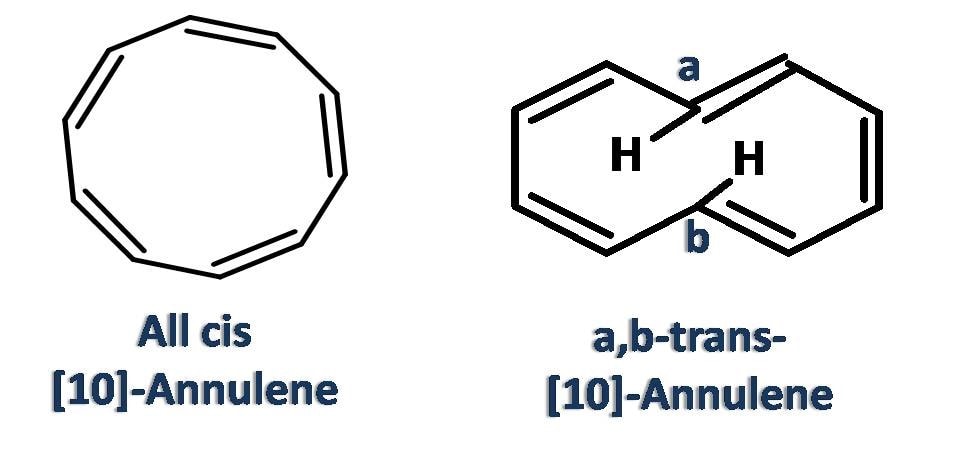
Now [10]-annulene contains 10 pi electrons but still it is not aromatic as the ring is not perfectly flat and small bend is observed in the structure due to repulsion of hydrogens.
Finally [14]-annulene and [18]-annulene are again aromatic as their hydrogens are farer apart and therefore negligible ring strain.

That’s about annulenes, monocyclic conjugated ring systems. Among these even membered annulenes are many of the times aromatic and more stable than corresponding odd membered annulenes.