What are activating and deactivating groups
by egpat Posted on 24 Apr 2020
If we compare the relative reactivity of the aromatic compounds towards electrophilic substitution reactions, which will be more reactive? Whether phenol or benzoic acid? Practically, the reactivity is observed more with phenol than with benzoic acid as the former has activating group while later is having deactivating group.
Why these two compounds behave differently, even they show similar mechanism of reaction? All that is due to tiny groups that already present on the benzene which control both the speed and yield of the reaction. Phenol has hydroxyl group (-OH) which promotes the reaction while benzoic acid has carboxylic acid group (-COOH) which reduces the reactivity acting as deactivating group. So, here let’s go in detail if such activating and deactivating groups and how they affect rate of reaction of aromatic compounds.
Effect of group on aromatic ring
In order to understand, the effect of group that already present on the aromatic ring on rate of reaction, let’s see the important step in the mechanism of electrophilic aromatic substitution. Aromatic compounds undergo electrophilic substitution by three steps.
Even though first step is not separated with next step, for convenience let’s see what happens in this step. In this there is a generation of electrophile which is then attacks the aromatic ring system to produce an intermediate in second step.
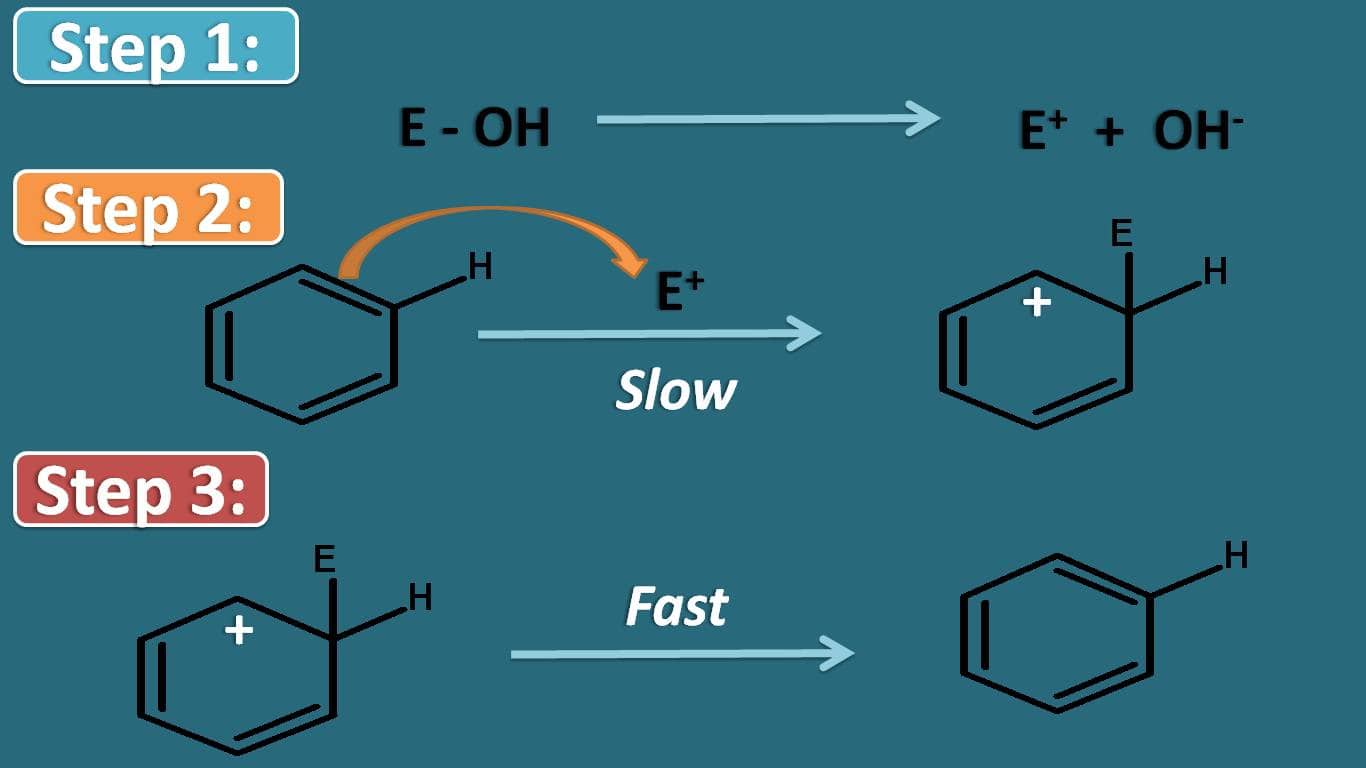
Finally one of the proton is abstracted to produce the substituted aromatic product.
Among these steps, the stability of arenium ion controls the overall rate of the reaction. So if the group already present on the ring may increase or decrease the stability of this intermediate.
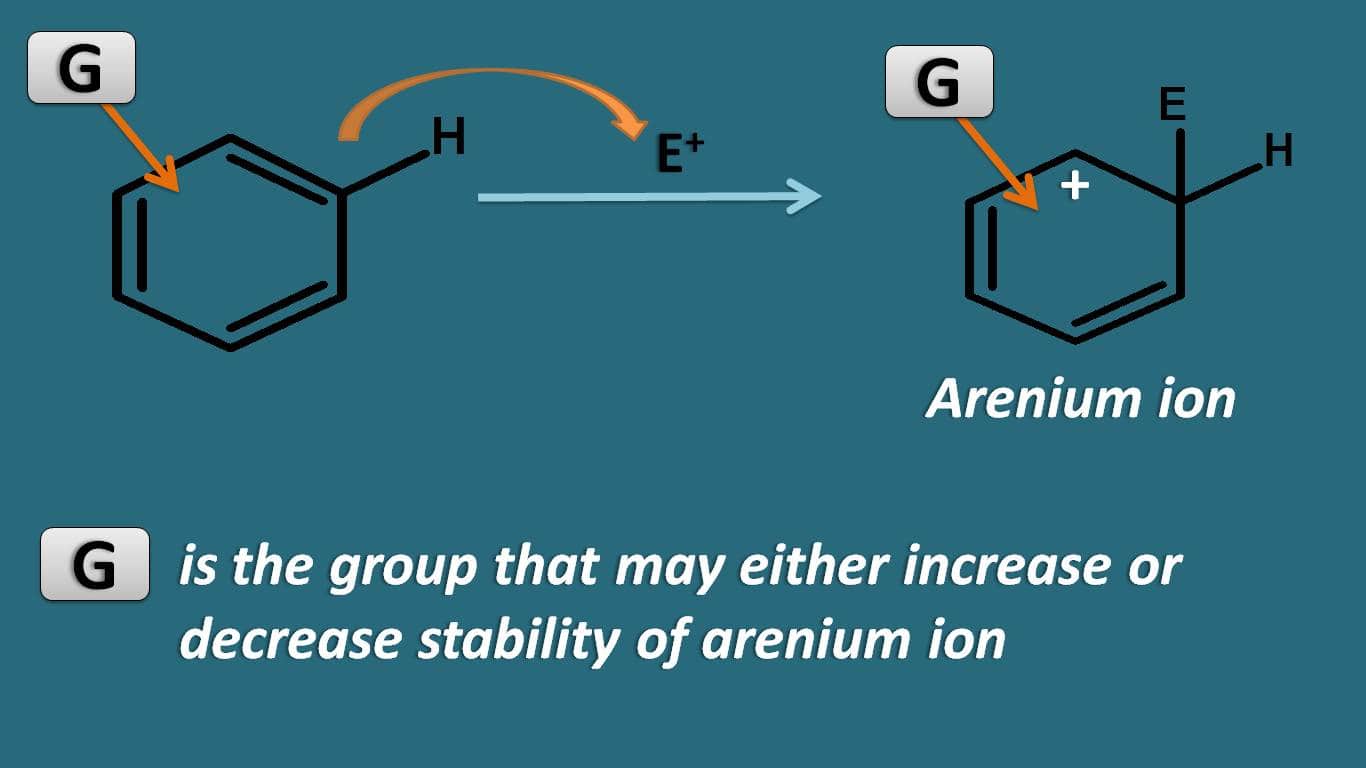
Now let’s see how various groups can affect the stability of this intermediate.
Activating and deactivating groups
If a group increases the stability thereby increases overall reactivity of the electrophilic substitution reaction, then it can be termed as activating group.

Similarly if a group reduces the stability of intermediate thereby reduces the reactivity, then it can be termed as deactivating group.
Which type of groups can act as activating groups? In order to stabilise the intermediate a group is required which promotes the delocalisation of positive charge.
Let’s take one example. Suppose the parent compound is phenol. Now how this –OH group can affect the stability? Whether it acts as activating or deactivating group?
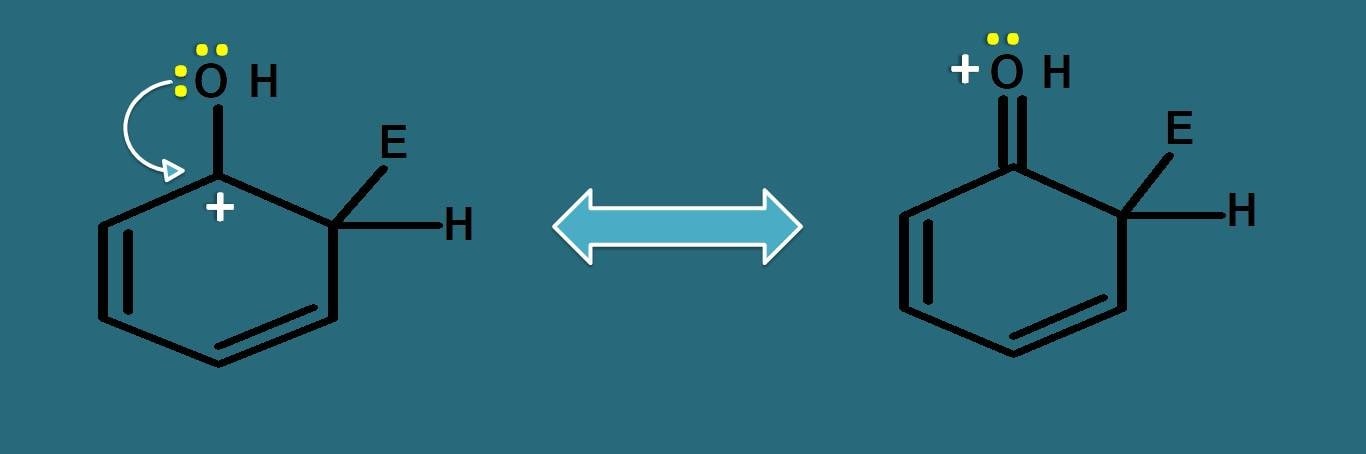
If we observe the resonating structures of intermediate, the positive charge can be further delocalised by –OH group due to presence of lone pair of electrons on oxygen.
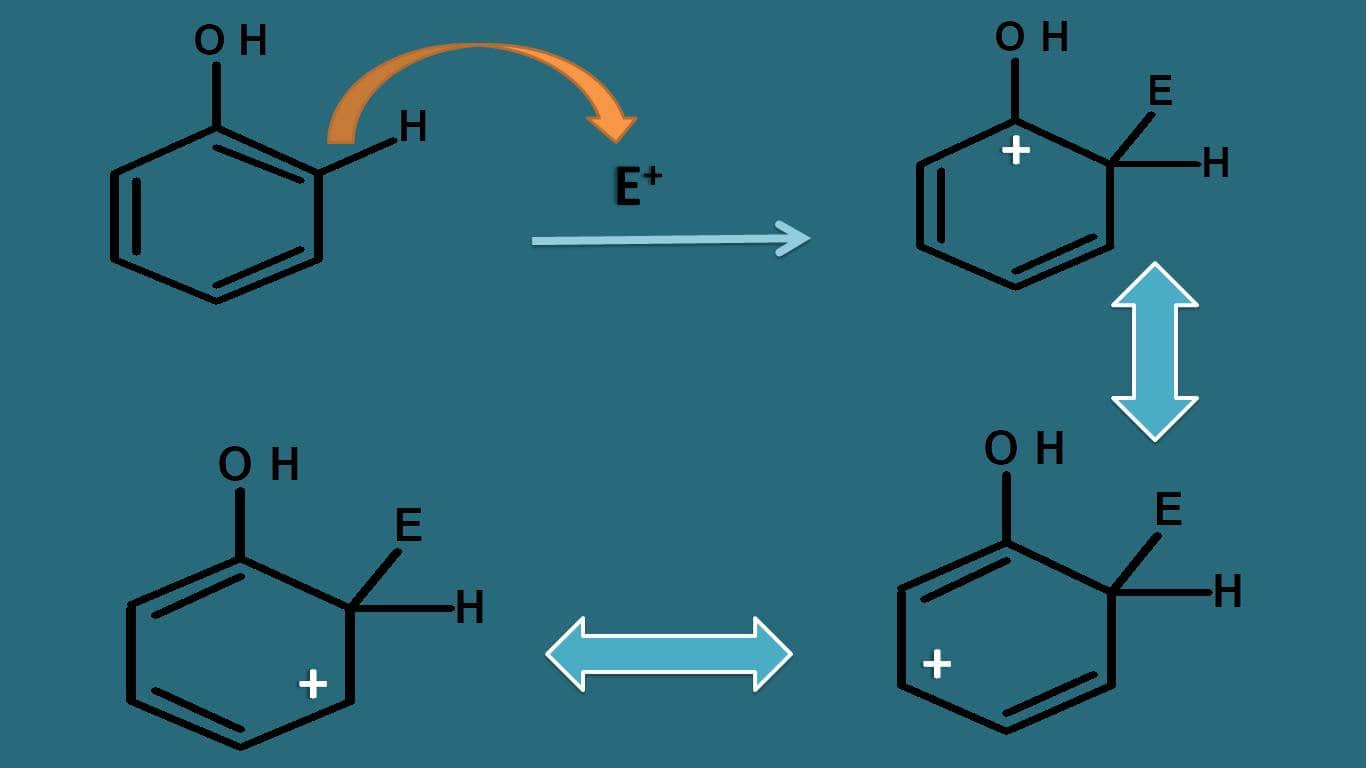
Even oxygen is electronegative, still it can participate well in resonance due to lone pair of electrons, therefore it can increase the stability of arenium ion. That’s why –OH acts as activating group.
Now let’s consider another like benzoic acid. It has carboxylic acid group and it has more electronegative oxygen attached to electropositive carbon by double bond. Due to presence of pi bond here resonance acts in opposite direction.
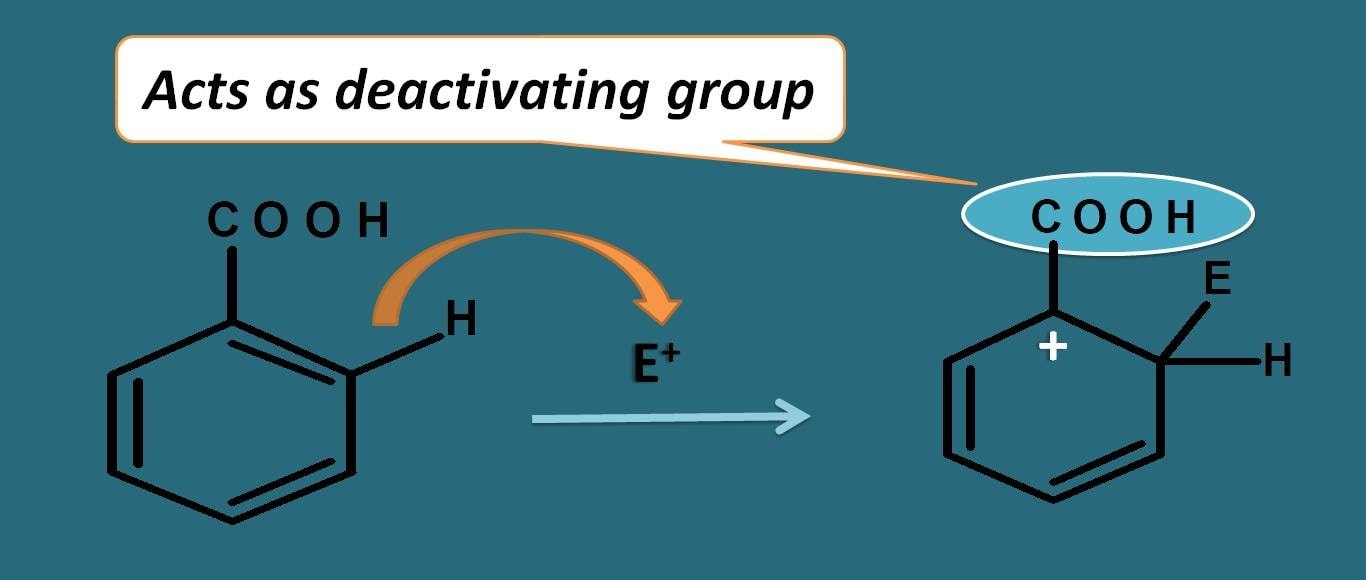
Oxygen due to electronegativity, it will take the pi bonds making carbon electropositive. This will pull the electrons further attached between the carbons and due to this the positive charge on the arenium ion is further increased rendering it less stable.
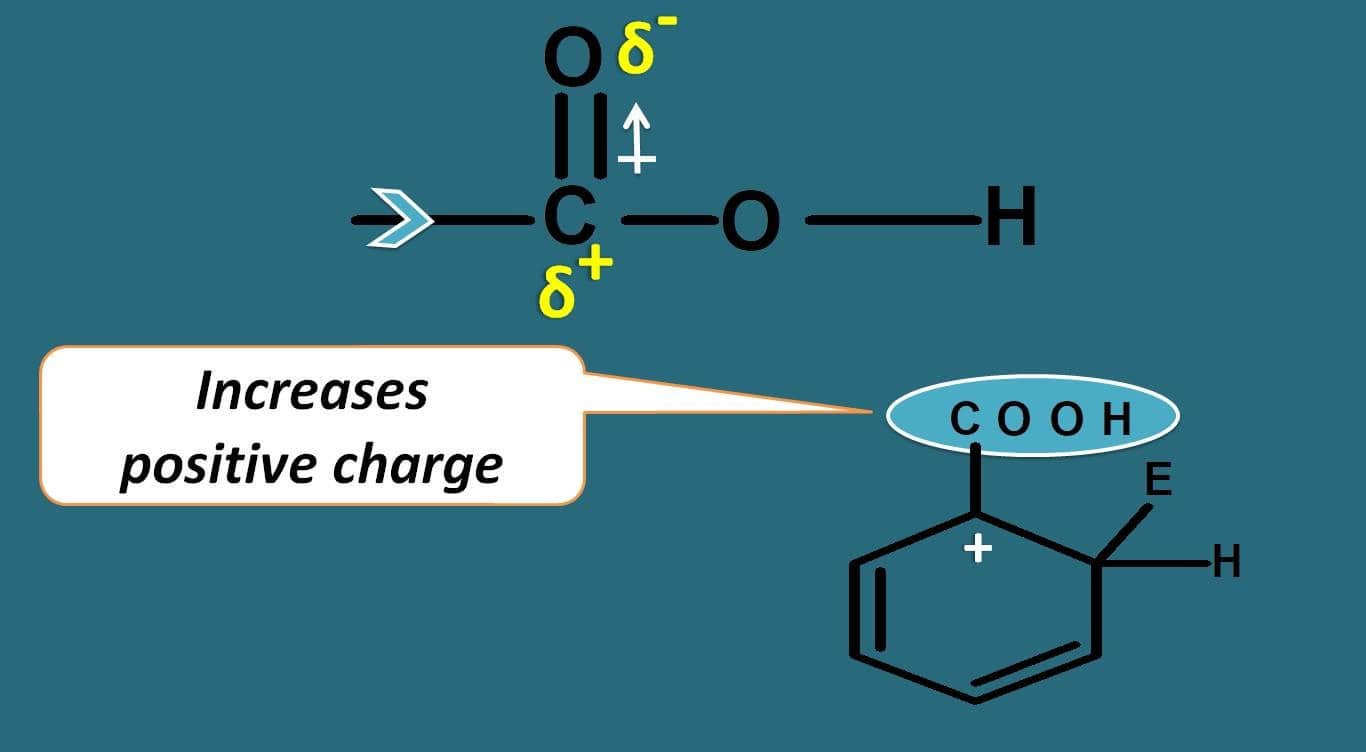
In this way, carboxylic acid acts as deactivating group. So in simple way, except halogens, all electron donating groups act as activating groups while electron withdrawing groups act as deactivating groups.
But, how same oxygen in –OH acts as activating group but in –COOH as deactivating group?
Normally when oxygen is attached by single bond just like in –OH, it acts as activating group by donation of lone pair of electrons. But when the oxygen is attached by double bond just like carbonyl group (-C=O) then it withdraws the electrons thereby acts as deactivating group.
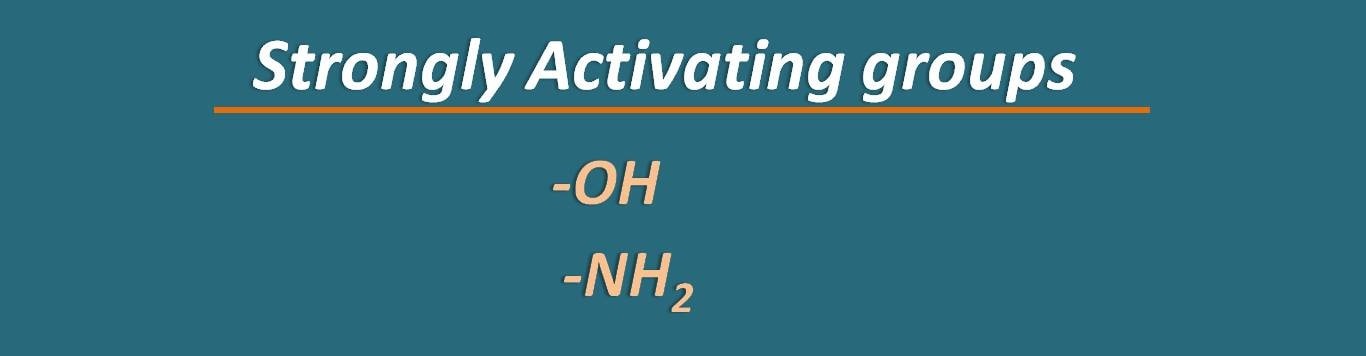
Now we can list out the groups as activating and deactivating groups. Among these –OH and –NH2 groups can be classified as strongly activating groups.
If the heteroatom in these groups is substituted with other groups, then the activating capacity is reduced therefore they can be categorised as moderately activating groups. For instance, in groups like –OCH3, –OC2H5 , the presence of alkyl group on oxygen slightly reduce its electron donating tendency.

Finally simple alkyl groups such as methyl and ethyl can donate the electrons due to positive inductive effect thereby act as weakly activating groups.
Img
Similarly all the functional groups that have a double or triple bond between carbon and heteroatom can act as deactivating groups.

Nitro group can also act as deactivating group due to presence of oxygen.
Exception of halogens
Till now we have seen that a group with electron donating capacity acts as activating and a group with electron withdrawing capacity acts as deactivating group. Then what about the halogens?
They can act as electron donating due to presence of lone pair of electrons and at the same time they can act as electron withdrawing group due to strong electronegativity of halogens.
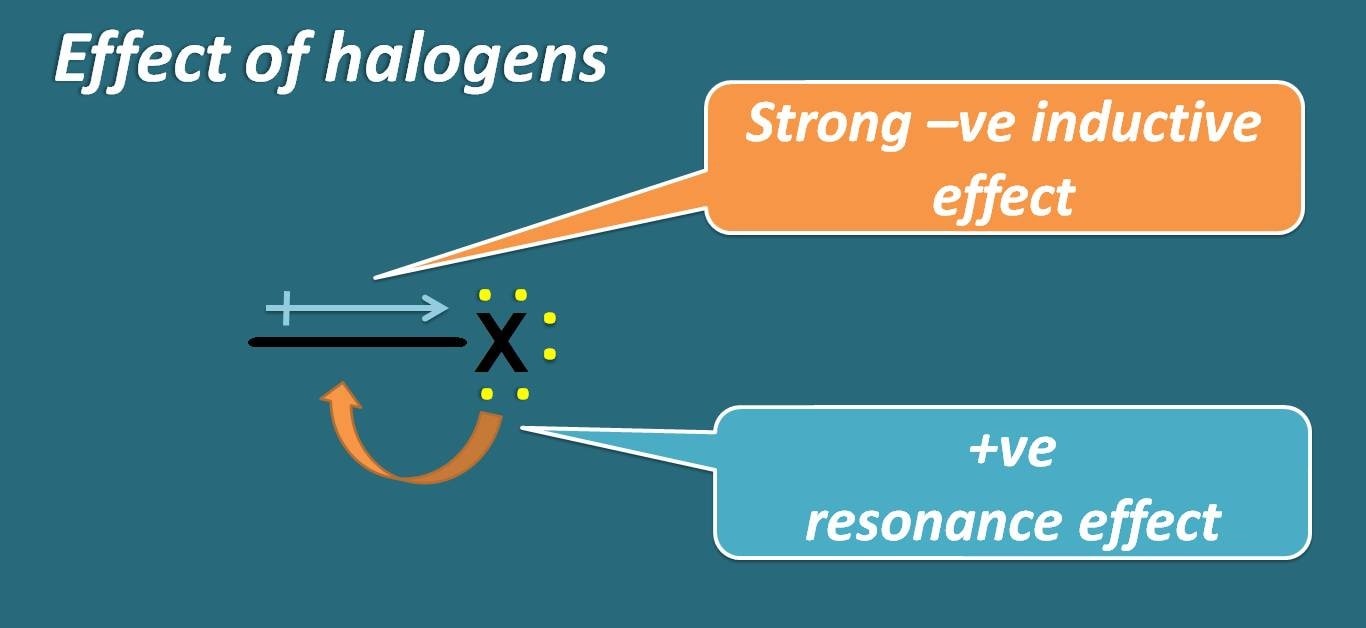
Who wins in this game? Whether resonance or inductive effect? Undoubtedly, its inductive effect predominates over resonance. So, halogens are treated as deactivating groups.
Even all the deactivating groups orient the incoming group to meta position, but still halogens orient the incoming group to ortho and meta positions.