Structural activity relationships of benzodiazepines
by egpat 05-10-2017
Benzodiazepines are well known sedative-anxiolytics widely prescribed in various anxiety disorders. These drugs are also called as minor tranquilisers as they produce sedation and increase calmness in the patients. They also induce sleep and increase overall sleep quality. All these actions of benzodiazepines can be attributed to their structure with little change in few drugs. We will see here how a small structural change can affect the pharmacodynamic and pharmacokinetic properties of these drugs.
Why SAR is important?
Studying of structural activity relationships (SAR) is very important to develop drugs with more selectivity to the target or more potent or more active. Sometimes, SAR can also be used to improve duration of action and bioavailability. It can be used to prepare a series of drugs with common structural features prove to have significant activity. So let’s discuss the SAR of benzodiazepines.
Structural activity relationships
The common structural feature of benzodiazepines involves six plus seven numbered fused ring system with two nitrogens.
Numbering is started from adjacent nitrogen to the bridge heads and second nitrogen is given 4th position. That’s why these drugs are called 1,4-benzodiazpines.
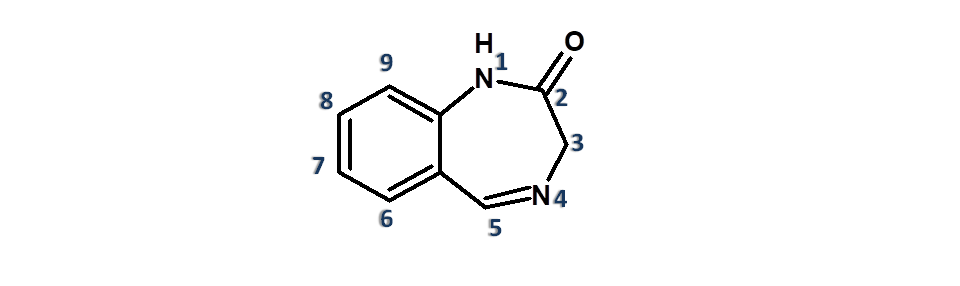
For more details, see nomenclature of benzodiazepines.
Now let’s see few of the structural modifications of benzodiazepines at various locations and their relation with activity.
1st position
Let’s start with first position.
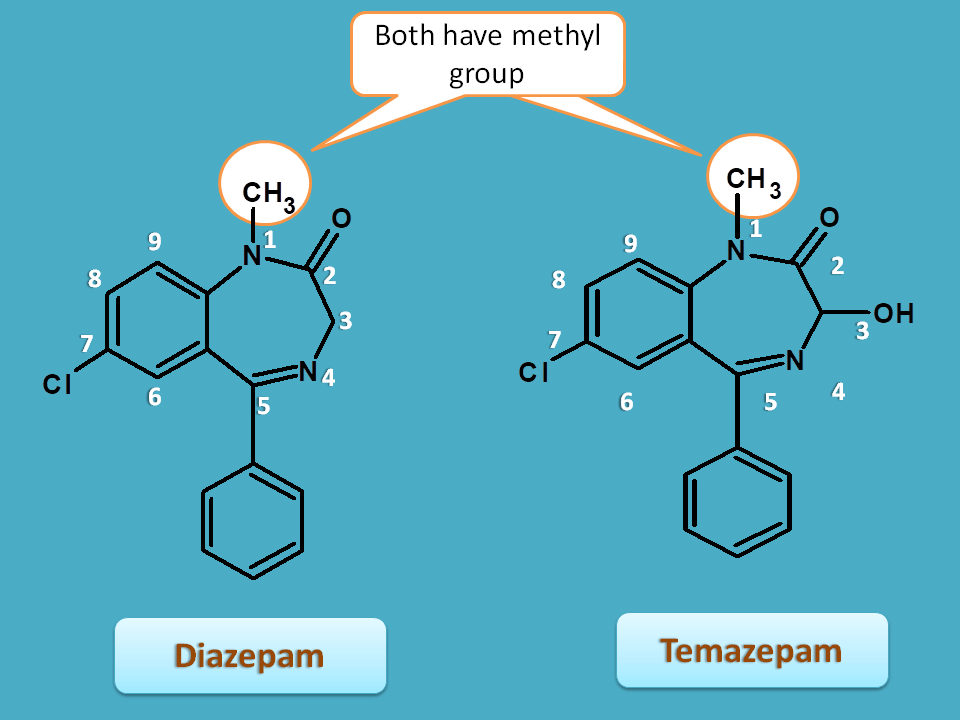
At 1st position, a small alkyl group is optimal for activity.
For example, diazepam and temazepam has methyl group at 1st position.
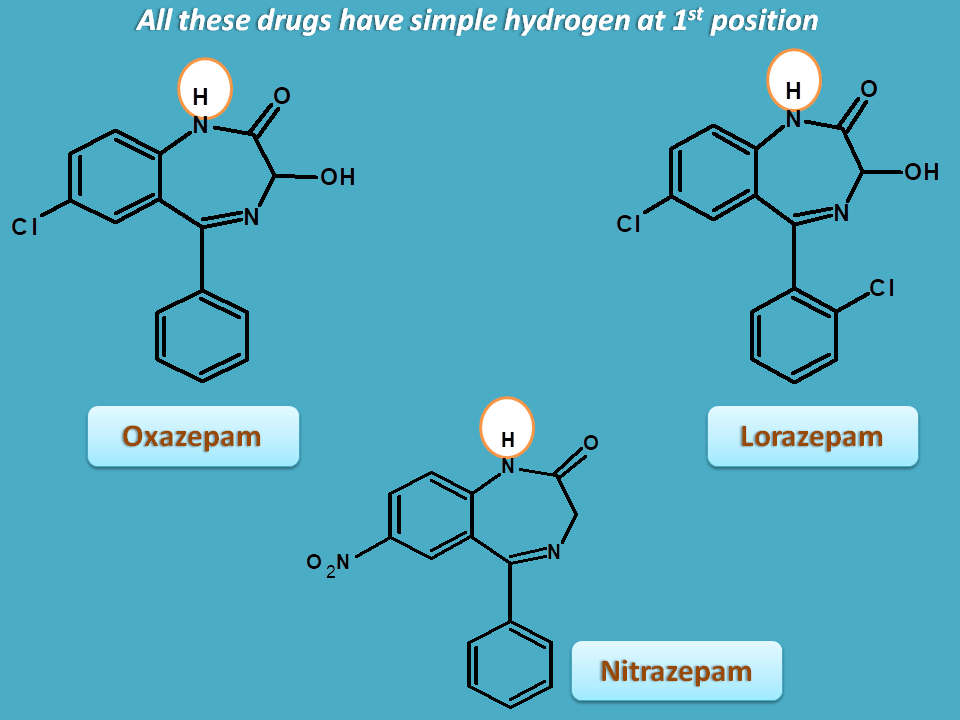
Alkyl group is not essential as few of the benzodiazepines don’t have any alkyl at 1st position. For example, benzodiazepines like oxazepam, lorazepam and nitrazepam simply have hydrogen at 1st position.
Similarly flurazepam has diethylaminoethyl group but it doesn’t add any advantage to their action.
2nd position
Second position is very important in view of pharmacological activity as the drugs bind to benzodiazepine receptor through this group.
A carbonyl group at 2nd position is essential for activity
You can easily observe this group in many of the benzodiazepines such as diazepam, oxazepm etc.
Here we can found two exceptions. First is at chlordiazepoxide and second at fused benzodiazepines.
Fused benzodiazepines like alprazolam, midazolam and triazolam interact with GABA receptors through triazole or imidazole ring. All other benzodiazepines require a keto group at 2nd position.
Chlordiazepoxide is the first benzodiazepine that was developed and it doesn’t have keto group at 2nd position.
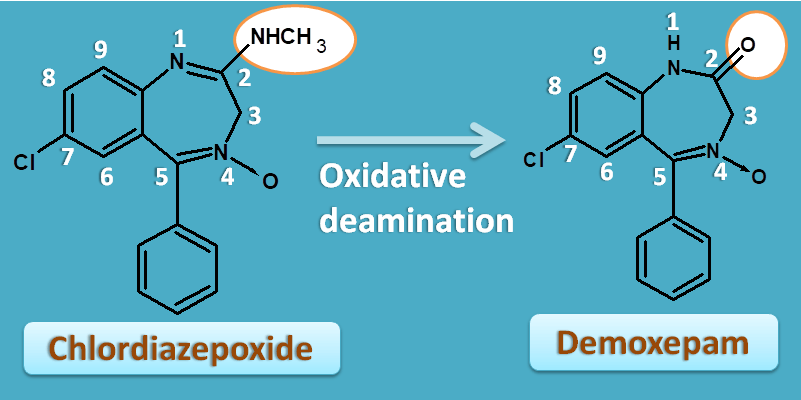
But in-vivo it can undergo oxidative deamination to produce demoxepam with keto group at second position.
This again proved that keto group at 2nd position is essential for activity.
3rd position
This position is very important in view of pharmacokinetics. It is obvious that drugs or metabolites which are highly polar can undergo direct conjugation and hence directly excreted.
A polar functional group at 3rd position increases excretion thereby decrease duration of action.
Drugs like lorazepm, oxazepam and temazepam have hydroxyl group at 3rd position making all these drugs polar and easily excretable. Hence all these drugs have short duration of action.
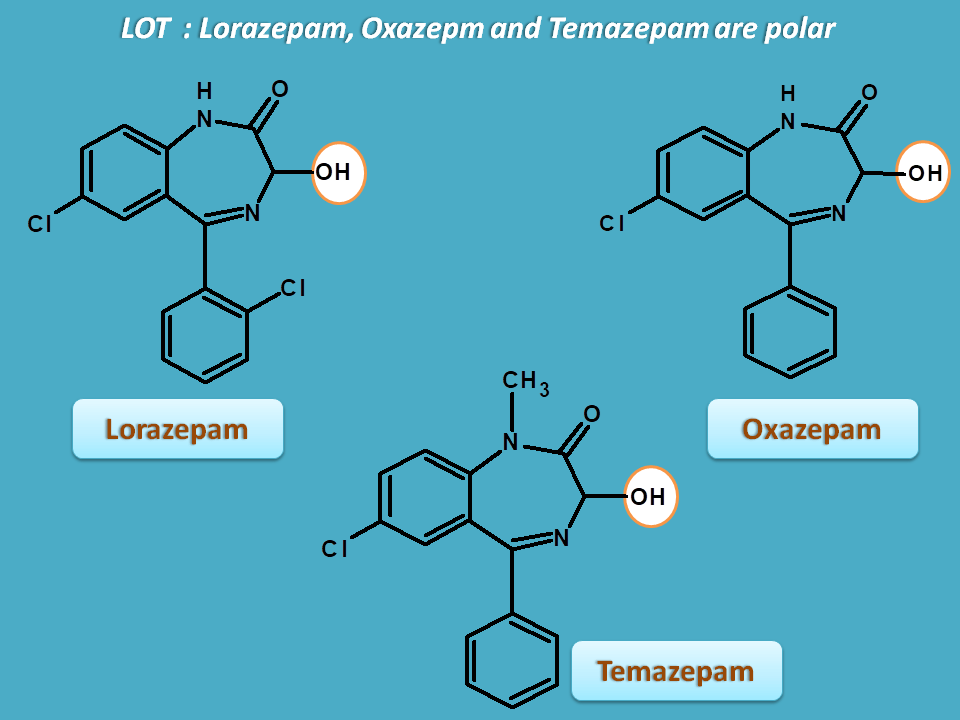
Oxazepam and lorazepam are highly polar and can be excreted without phase I metabolism.
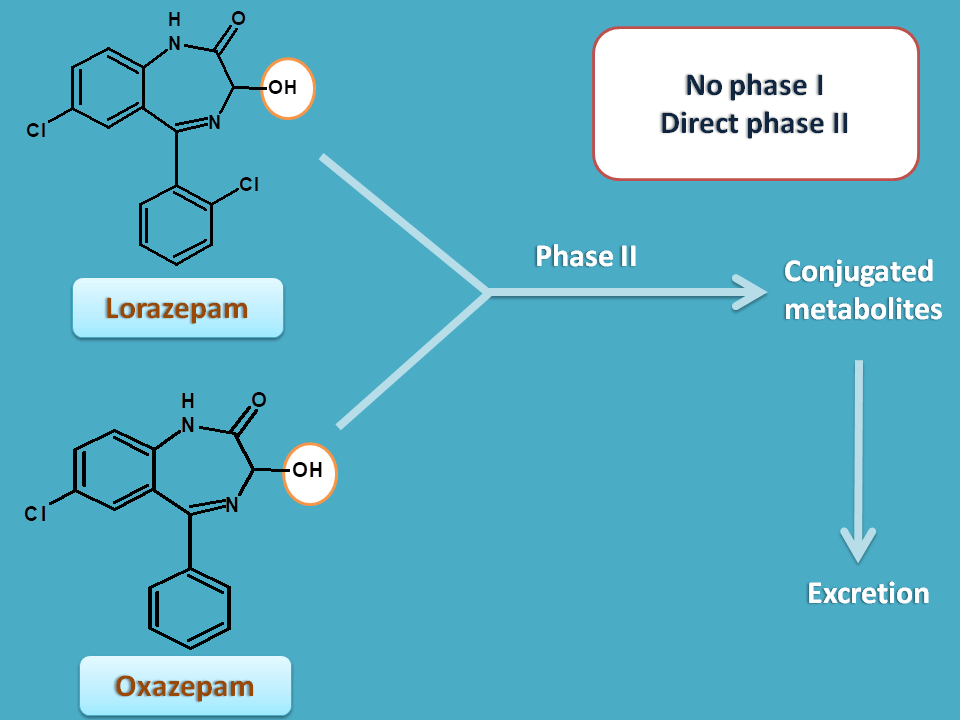
Temazepam also shows a little phase I reaction. It undergoes demethylation and converted into oxazepam and then excreted.

4th position
In all benzodiazepines we can observe an unsaturation at 4th and 5th position. This is essential for activity and saturation of this double bond may decrease the activity. Even shift of the double bond to 3rd and 4ht position decreases the activity.
5th position
A simple aromatic ring like phenyl group is optimal for activity. Again all benzodiazepines including fused benzodiazepine ring systems have a phenyl group at 5th position.
For example, drugs like diazepam, nitrazepam, oxazepam, temazepam and chlordiazepoxide all have a phenyl group at 5th position.
Substitution on phenyl group also plays a key role in influencing activity. But all positions may not yield favourable results. Those drugs having phenyl group with ortho or diorhto substitution with an electron withdrawing group found to increase activity. At the same time, para substitution decreases the activity.
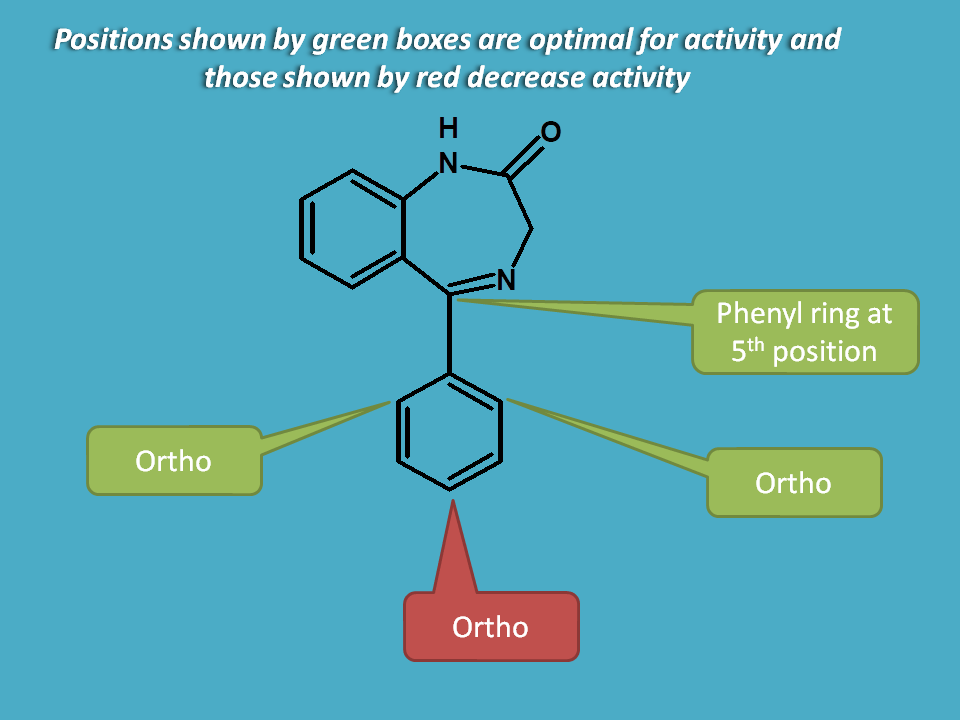
We can easily observe this in the names of few benzodiazepines.
For example, flurazepam has fluoro group and clonazepam has chlorine group
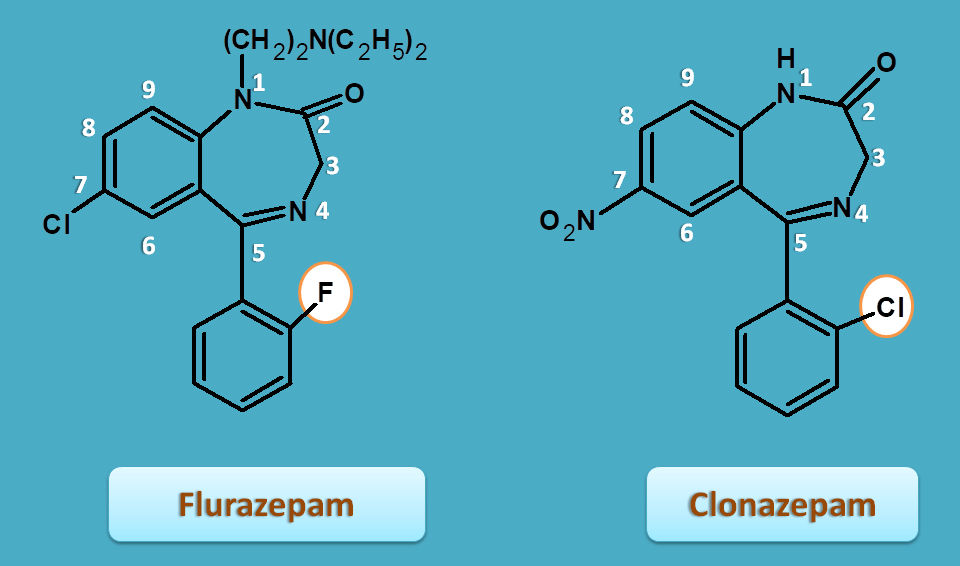
Similarly other drugs like triazolam and midazolam has chlorine and fluorine groups respectively at ortho position of the phenyl ring.
7th position
This is again very important position to determine the potency of benzodiazepine. Electron withdrawing groups like halogens or nitro group increase the activity.
Higher the electornegativity higher the potency
Therefore nitrazepam is more potent than diazepam.
What else?
We left with positions 6,8 and 9. Any substitution on these groups may decrease the activity. Hence these positions should not be substituted.
Fused benzodiazepines
Benzodiazepine ring may be fused with triazole or imidazole ring to produce triazolobenzodiazepine and imidazolobenzodiazepine respectively.
Here the numbering starts from either triazole or imidazole ring adjacent to bridge heads. Due to this normal positions of the benzodiazepine ring are shifted by one number.
The same SAR also applies in these compounds at relative positions. As the numbering was changed here, the relative positions are shifted by one number.
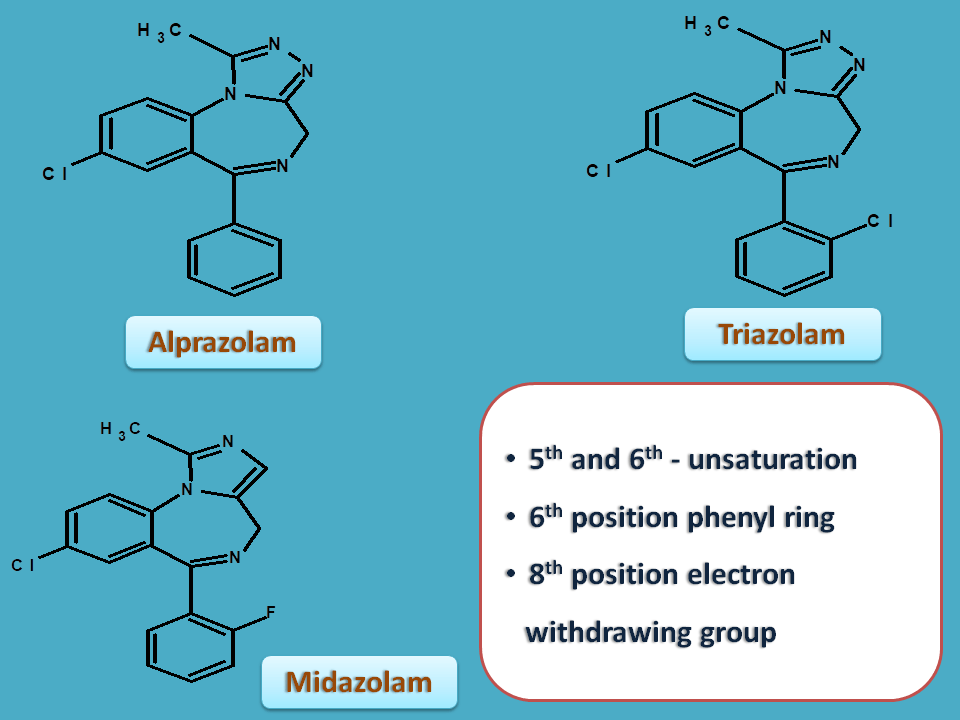
Hence the optimal groups are
- 5th position – unsaturation
- 6th position - phenyl ring
- 8th position – electorn withdrawing group
We can find all these features in alprazolam, triazolam and midazolam.
Alprazolam is one of the drugs widely used for anxiety disorders. It is a medium acting benzodiazepine with duration of action as 24 hrs. Hence it is given once a daily.
Triazolam is a ultra short acting benzodiazepine with duration of action less than 6 hrs. Due to short action it can be used as hypnotic.
Midazolam is not used anxiolytic but used in induction of anaesthesia by IV route.