Solvatochromism - The effect of polarity of solvent on lamba max
by egpat 05 Jun 2019
We use spectroscopy to identify as well quantify the analyte molecules by observing their absorption or emission. Do we directly place the sample in the cell? No, many of the times we need a solution of the analyte dissolved in a suitable solvent. Then, does the solvent have any effect on the spectra o analyte? Well, it may have an effect on the spectra – commonly known as solvatochromism. So let’s discuss here how will be the effect of solvent and what happens to spectra.
Selection of solvent
Most of the analytes use UV-visible radiation for routine analysis and in that situation we have to select a solvent which is transparent and not interfering with the absorption or emission of analyte.
Then, how it can be selected?
First, let’s think about analyte. If an analyte absorbs UV-visible radiation, it should absorb anywhere within the region from 200 to 800 nm. Electronic transitions of π → π* and n→ π* are mainly responsible for absorption in this region.

Now, the path is clear. Simply we can select a solvent which doesn’t absorb within this region and doesn’t undergo either π → π* and n→ π* transitions.

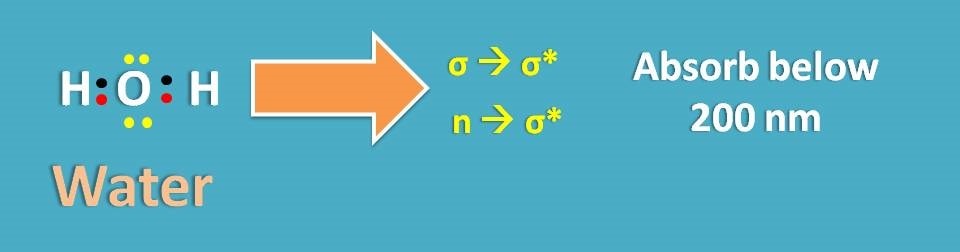
Stated in another way, the solvent should not have pi electrons. Let’s take simple case of water. It has only σ and n electrons but has no pi electrons. So it can show σ → σ* and n → σ* transitions both fall in a region of below 200 nm.
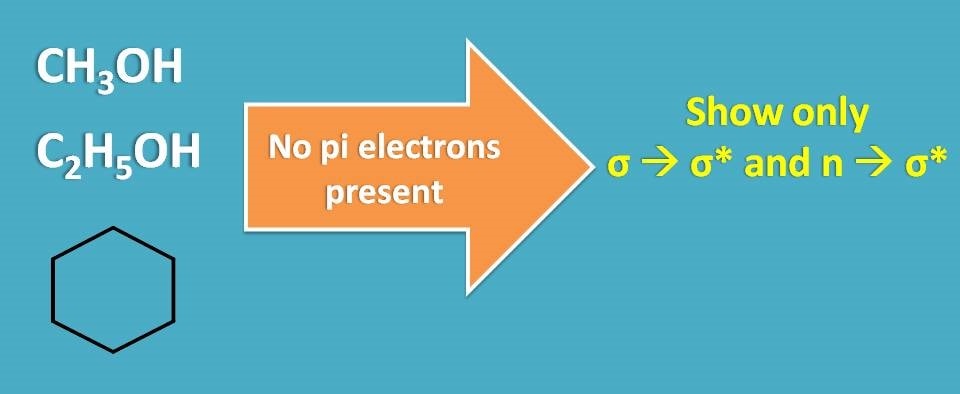
Similarly we can select any solvent which doesn’t have pi electrons. Solvents like methanol, ethanol, cyclohexane all can be selected as solvents.
In this way, a suitable solvent can be selected which do not interfere with the spectra of analyte. Then, does solvent have no effect at all?
Interestingly, solvent can affect the spectra even it does not absorb within the region where analyte absorbs. This all due to polarity of the solvent and let’s discuss it in detail here.
Polarity of the solvent
One of the important property of solvent to be considered is its polarity. As you know, polar solvent dissolves and stabilizes polar analyte. Since analyte can exist in either ground or excited state, the stability of any one of the states depends on their relative polarity.
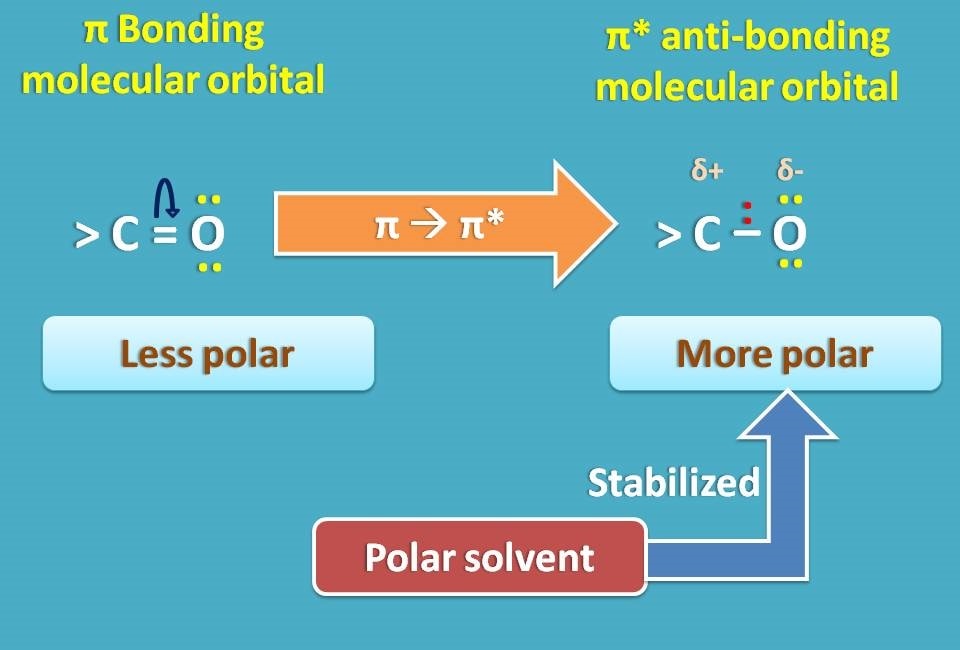
To understand clearly, let’s take π → π* transition. In this transition, the antibonding molecular orbital is more polar due to separation of charges than bonding molecular orbital.
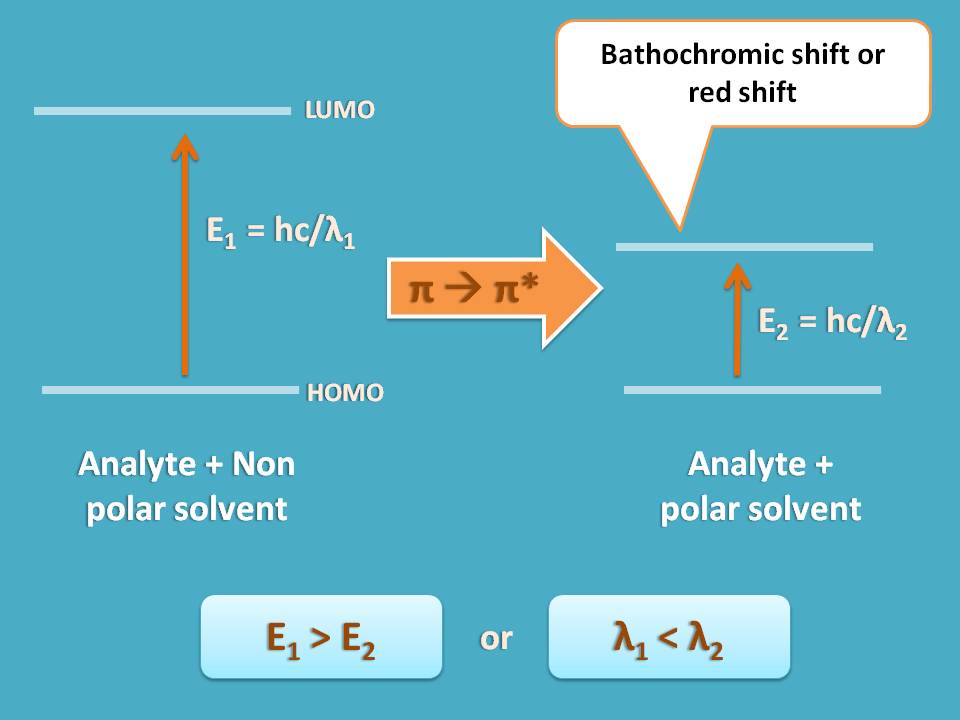
So if we use a polar solvent, it will stabilize the antibonding molecular orbital reducing the energy gap between π and π* molecular orbitals making this transition somewhat easy.
As we know that energy is inversely proportional to wavelength, if energy decreases, wavelength increases. Therefore a polar solvent decreases the energy gap for π → π* transition thereby increases the corresponding wavelength required for transitions.
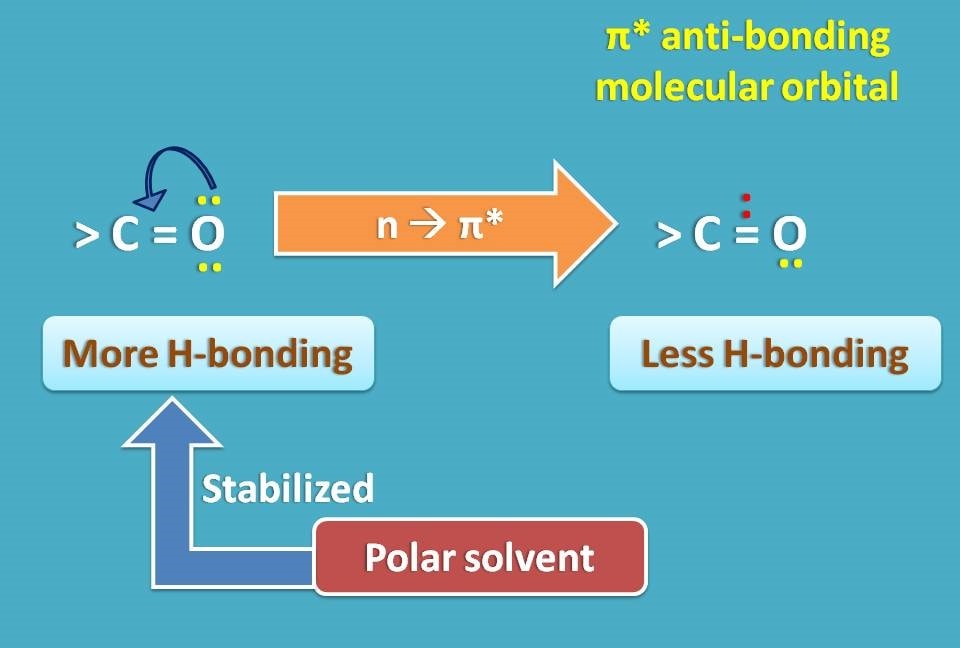
Now let’s turn to the case of n → π* transition. Here the n electrons are responsible for hydrogen bonding with water making the HOMO as more polar. When they converted into π* anti bonding electrons in LUMO they can’t form hydrogen bond, hence less polar.
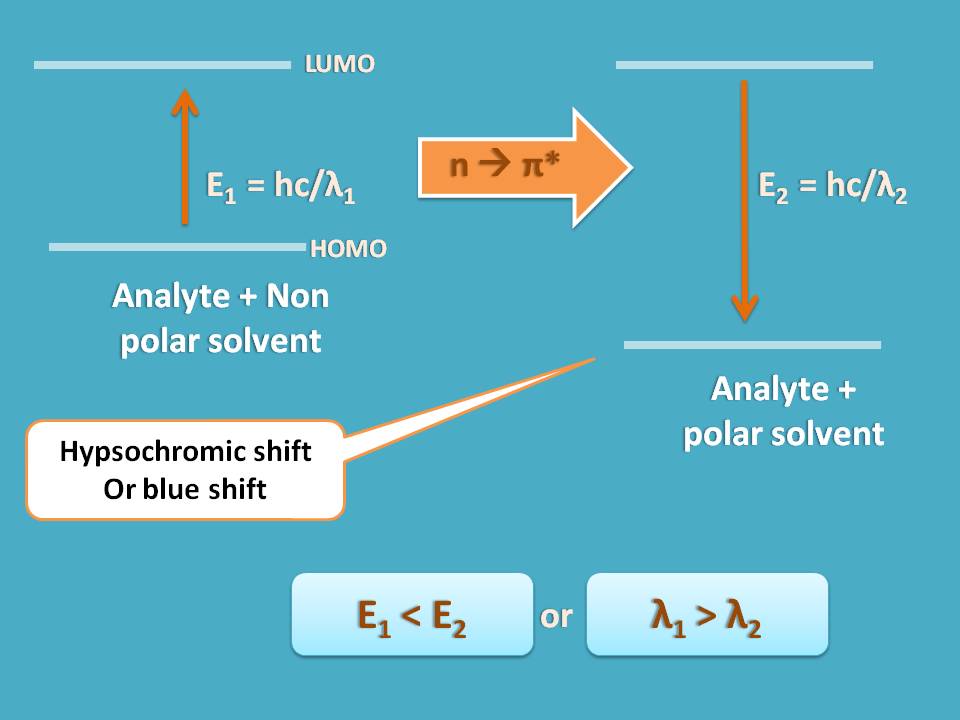
So for n → π* transition, a polar solvent stabilizes HOMO resulting in increase in the energy gap between HOMO and LUMO.
Positive and negative solvatochromism
As discussed above, a solvent can either increase or decrease the transition depending upon the type of transition involved. Accordingly we can define the increase in transition as positive solvatochromism and a decrease in the transition as negative solvatochromism.
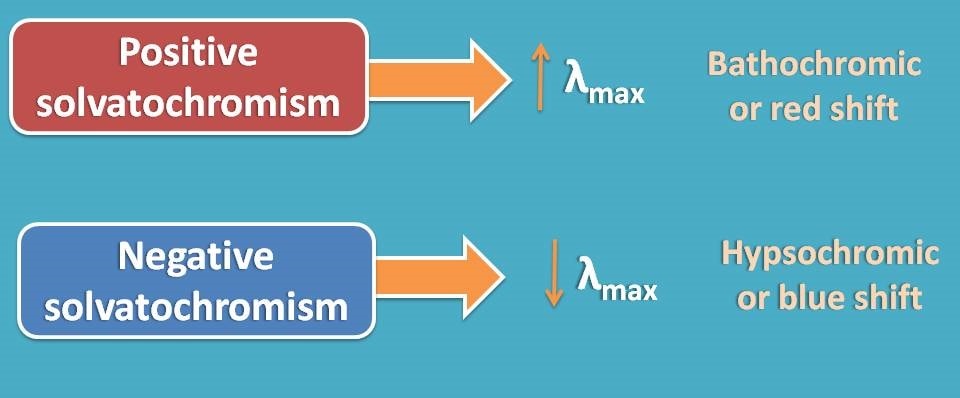
Positive solvatochromism results in bathochromic shift where λmax is increased as the energy gap between HOMO and LUMO is reduced due to polarity of the solvent. Similarly, negative solvatochromism results in hypsochromic shift where λmax is decreased due to increase in the energy gap between HOMO and LUMO.