IUPAC names of alkanes with examples
by egpat 30 Mar 2019
Carbon plays a central role for making backbone of many compounds in organic chemistry. Interestingly, its valency is four and if it is attached with four hydrogens it forms simple hydrocarbon methane. Alkanes are the saturated hydrocarbons without any functional group so IUPAC naming is somewhat easy
So, let’s go with simple examples initially and then to more advanced structures of alkanes. If you are an advanced reader, you can jump to examples given at the end of this article.
Writing IUPAC name of alkane is very simple and direct method where “-ane” is used as suffix to indicate alkanes.
We can select a suitable prefix based on the number of carbons in the parent chain and can be combine with suffix to complete the IUPAC name of alkane.
For instance,
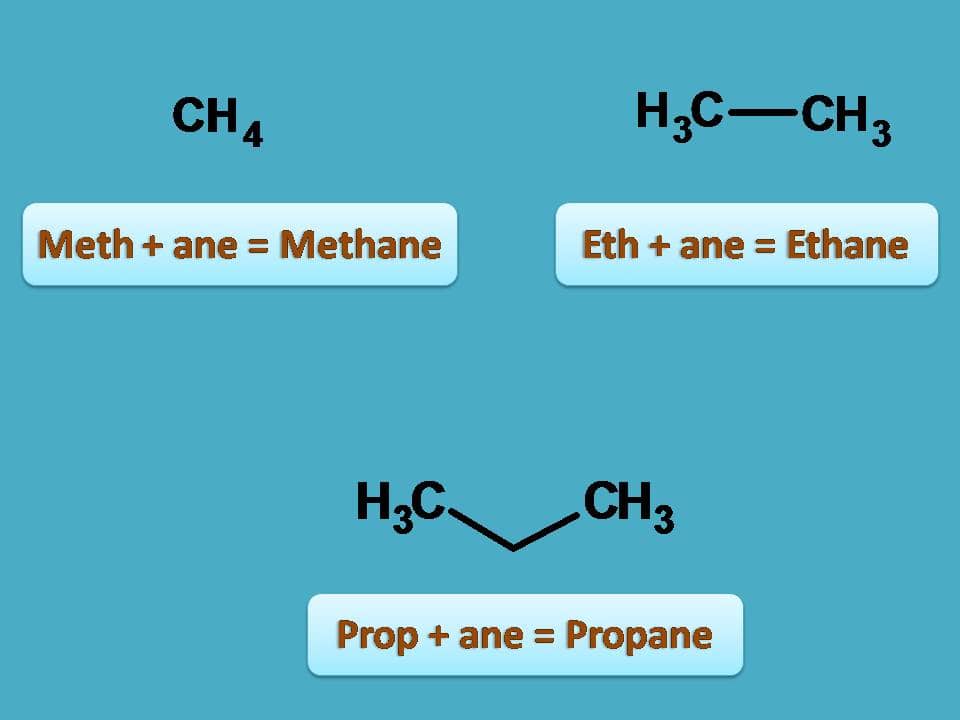
- Here first structure is Meth + ane=Methane.
- Second structure is Eth + ane=Ethane
- Third structure is Prop + ane=Propane
We can extend this with four carbon chain as butane.
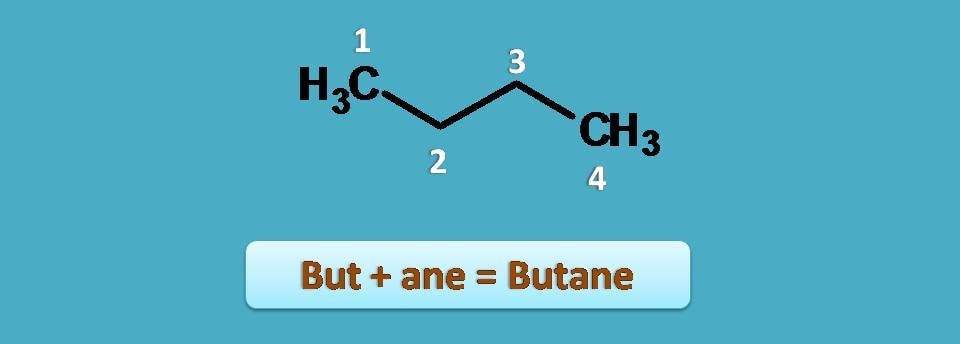
Of course, all the above cited examples are simple straight chain compounds without any branching. Now let’s go with alkanes having side chains.
Isobutane
Isobutane is the first alkane that has branching.
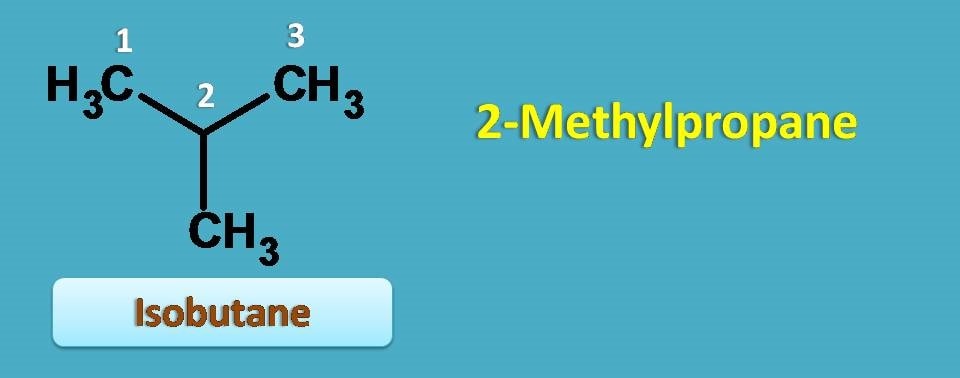
Even isobutane contains four carbons, according to IUPAC rules, we have to select longest chain as parent chain. Here it contains longest chain with 3 carbons methyl group being attached at second position. Hence IUPAC name of isobutene is 2–methylpropane.
Isopentane
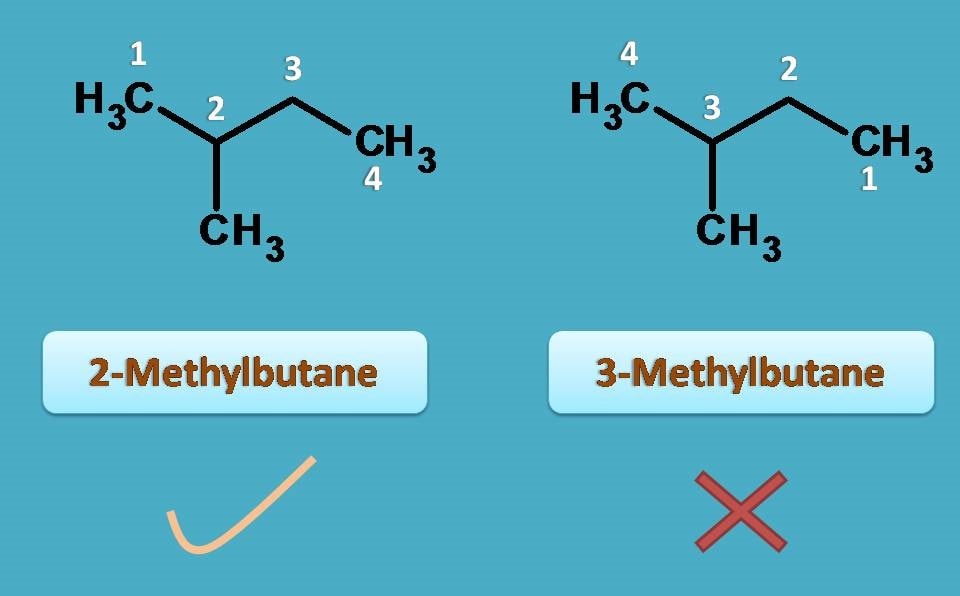
Here it has two longest chains and any one can be selected as parent chain. So if we select one of the chains, it contains four carbons hence prefix is “But-”.
Now methyl group is present as side chain at 2nd position hence prefix is 2-Methyl. Combining all these, IUPAC name of alkane in the above example is
2-Methyl + But + ane=2-Methylbutane
That’s fine, let’s make another step.
Neopentane
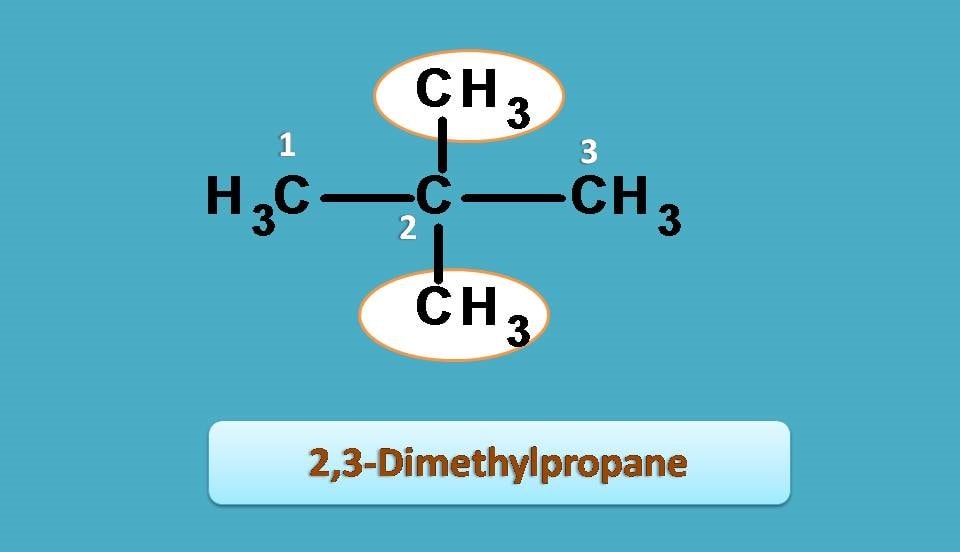
In case of neopentane, the longest chain contains only three carbons, hence the root name is “Prop-”. Other two methyl groups are considered as side chains which are attached at second position, therefore indicated by “2,2-Dimethyl”.
Combining all, IUPAC name of neopentane is 2,2-dimethylpropane
Higher alkanes
Now let’s go with more advanced examples and let’s apply all IUPAC rules to get the name.
Consider the following structure
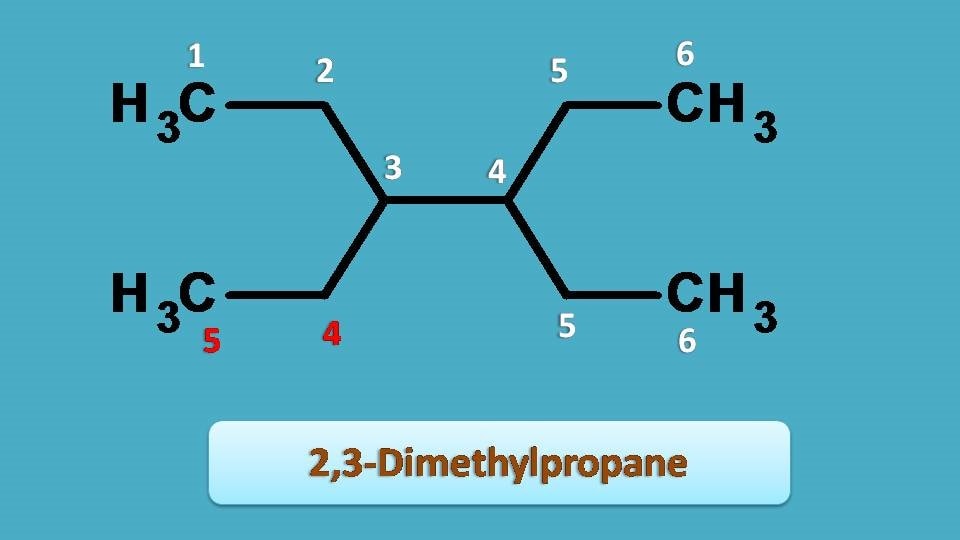
Since we are naming alkanes , our first role is to identify the longest chain. If you observe the structure, three types of chains are possible two chains each with 6 carbons and another chain with 5 carbons.
Then, which of the above chains should be selected as parent chain?
Obviously, the chain indicated by red colored numbering contains only 5 carbons hence not the longest chain and therefore not considered as parent chain.
Now, we have two options. Which is going to win?
Interestingly both the chains are similar having same side chains.
So, any one of them can be selected as parent chain. Going with one of that, now you can easily find that two side chains are present at 3rd and 4th positions, which are, of course, same and since they contain two carbons, we have to use prefix
3,4 + di + ethyl =3,4-diethyl
As the parent chain contains six carbons, the root name is ‘’hex”. Finally, combining all we can put the name as “3,4-Diethylhexane”.
So, simple it is, isn’t it? Let’s go further with naming of alkane having more branching.
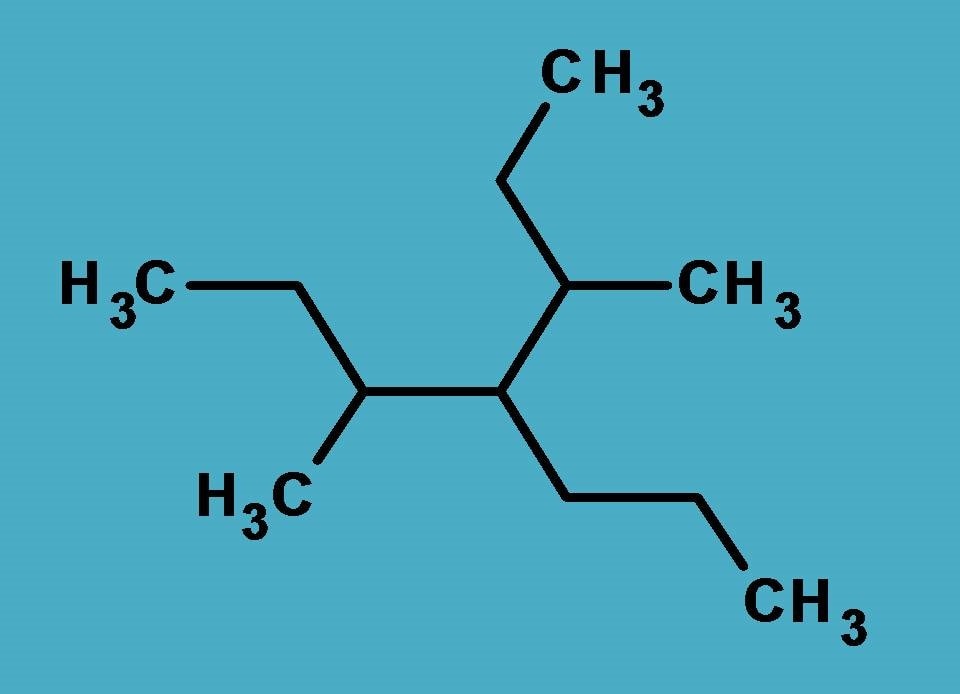
As with the previous example, here again we have to identify the longest chain and so many possibilities arise in selection of parent chain.
As indicated above, three longest chains are possible each with seven carbons, but again the same questions peeps into our mind, which should be selected as parent chain?
In contrast to previous example, all these longest chains are not identical hence only one of them will win the competition. Who is that and how can we find it?
Yes, here we have use a rule to select only one as the parent chain.
‘’the longest chain with more number of side chains should be selected as parent chain.”
Great, now the task is simple. You can easily find that chains numbered with red or yellow color have only two side chains while the chain numbered with complete white color has three side chains hence selected as parent chain.
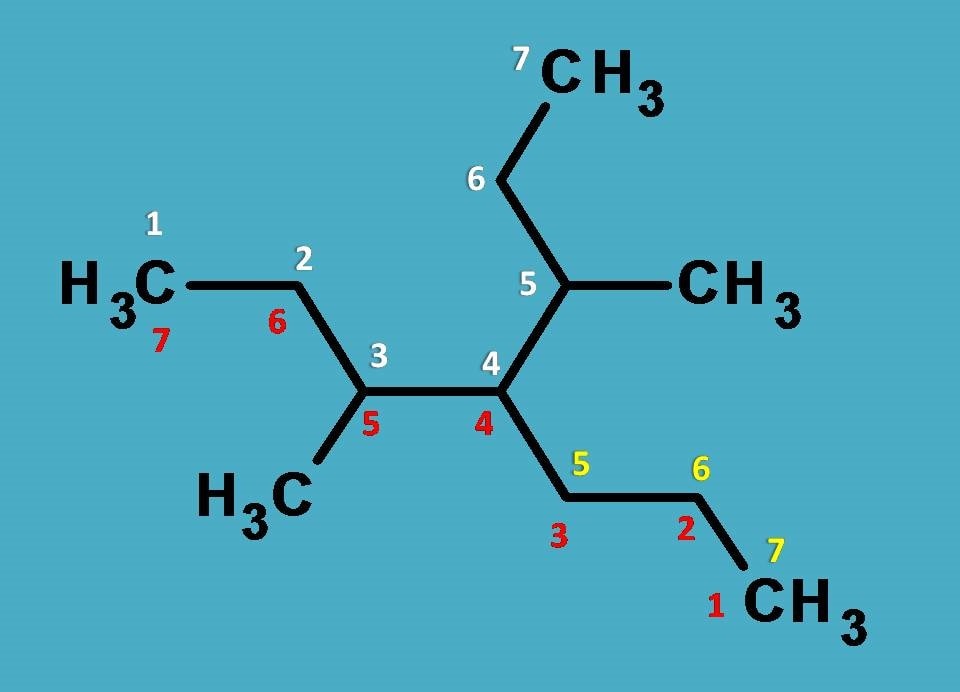
So, if you identify the side chains, two methyl groups are present at 3rd and 5th positions whereas propyl group is present at 4th position. Hence name of the side chains is
3,4-Dimethyl-4-propyl
As you are familiar, the root name is ‘’hept” as the longest chain contains 7 carbons.
Completing the name, it is
3,4-Dimethyl-4-propylheptane
Practical examples
Till now we have seen IUPAC names of simple alkanes, now let’s have various examples of compounds having functional group and different alkyl side chains.
Example 1
Let’s take first example.
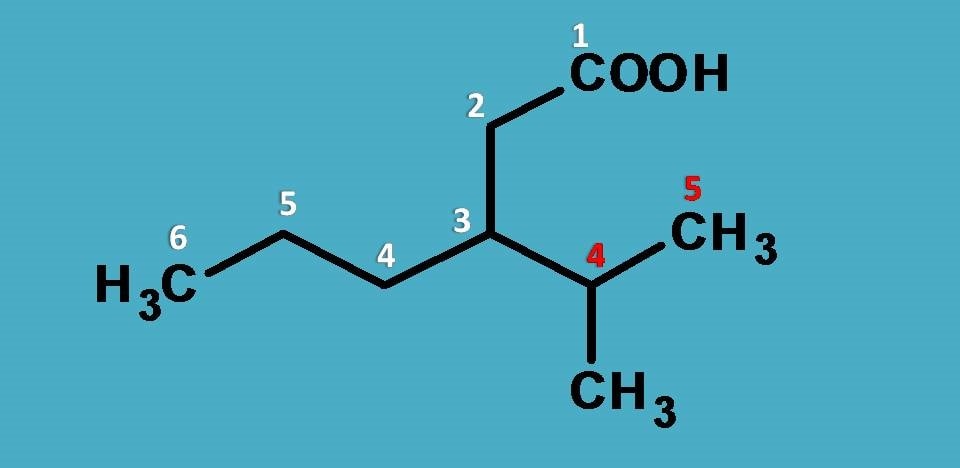
Here carboxylic acid is the principal functional group and we have to select longest chain including this group. So we can start numbering from carboxylic acid and if you count, two chains are possible one with 6 carbons indicated by white color and another with five carbons indicated by red color.
Which should be selected?
Definitely, it is longest chain with 6 carbons. Now it is turn of side chain. How to name it? Here side chain again contains side chain. Commonly we can name the side chain as isopropyl but strictly writing name by IUPAC, we have to provide numbering to the side chain at the point of attachment.
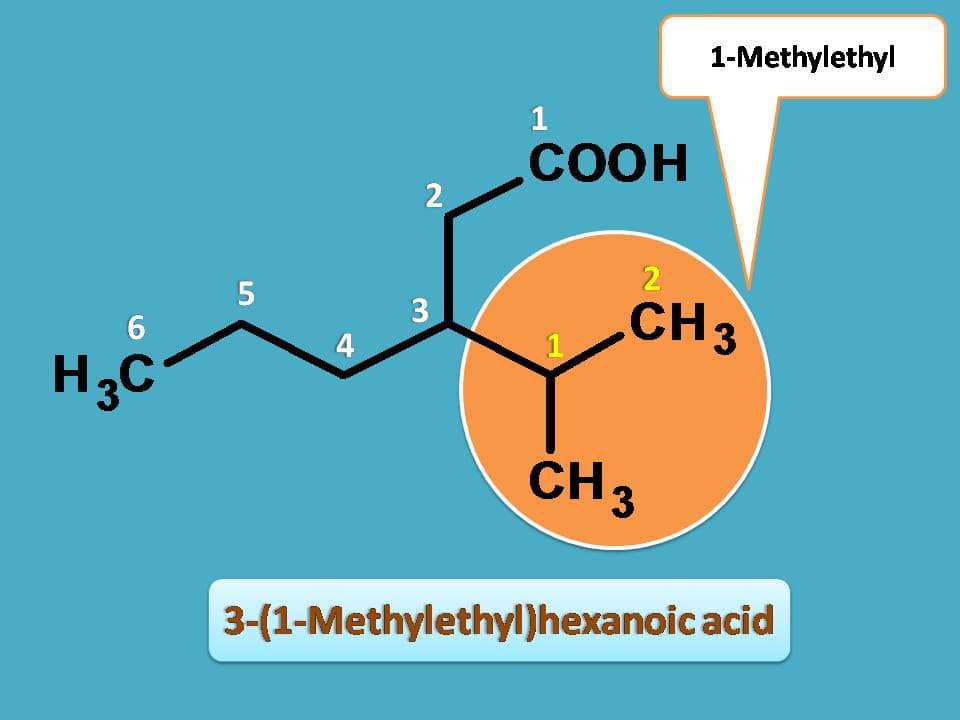
So, the side chain contains methyl group at 1st position therefore named as (1-methyl)ethyl. As this group is attached to main chain at 3rd position, the complete name of the side chain can be written as 3-[(1-methyl)ethyl]
Note: To differentiate numbering of side chain sometimes a prime is used in side chain numbering. So 3-[(1-methyl)ethyl] can also be written as 3-[(1’-methyl)ethyl]. But for practical purpose, we can omit prime notation and even few of the curved brackets. For instance, we can name the side chain simply as 3-(1-methylethyl).
Now, combining all name of the compound is
3-(1-methylethyl)hexanoic acid
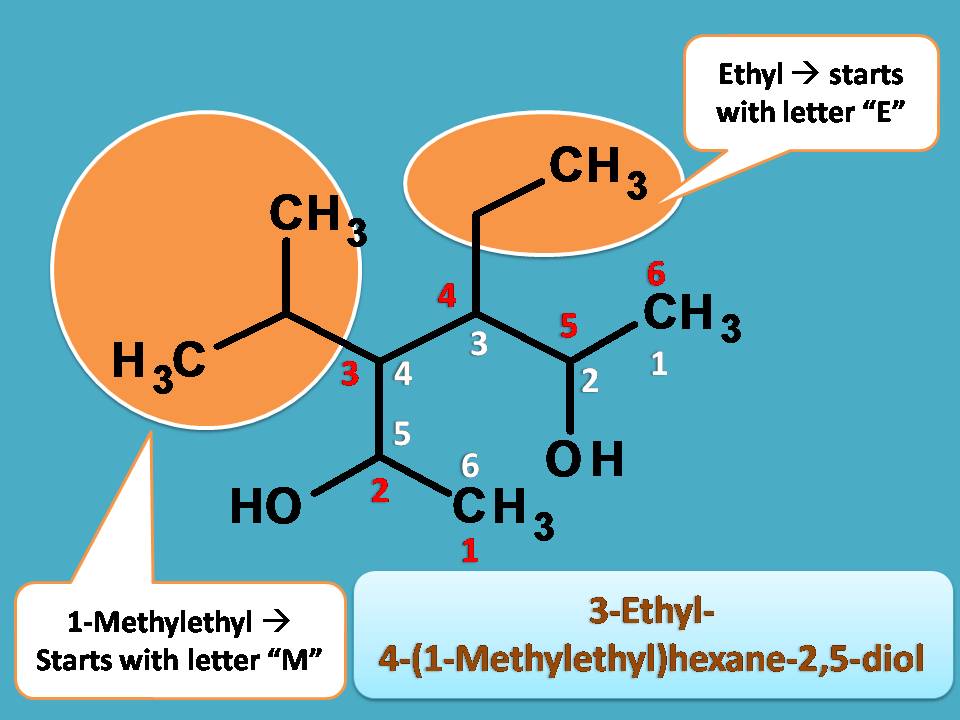
Example 2
Let’s go with another example.
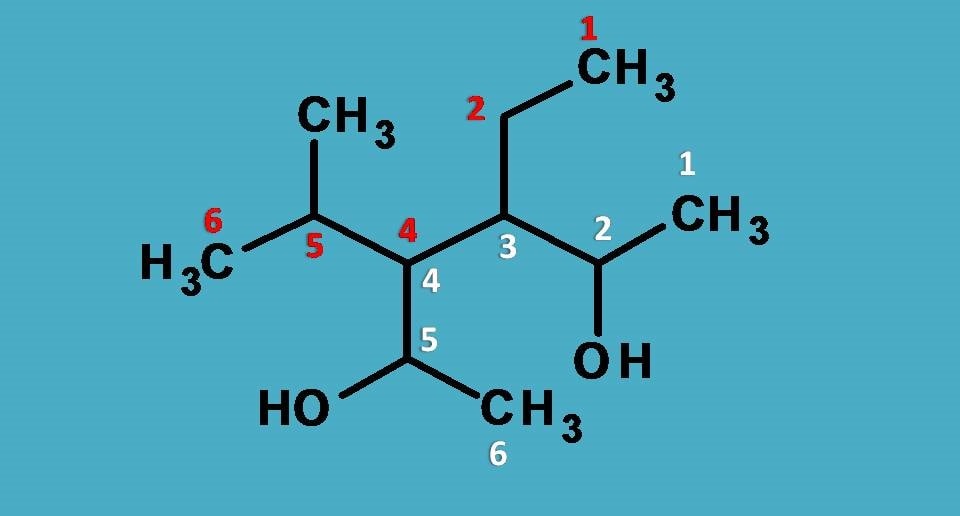
Here hydroxyl group is the principal functional group and two such groups exist there. Next step is to select longest chain. Two types of chains are possible each with 6 carbons.
Then, who is the winner?
Undoubtedly, the longest chain with more number of functional groups as indicated by white colored numbering here. The chain indicated by red colored numbering doesn’t contain any functional group.
Since the longest chain contains 6 carbons, the root name is “Hex-”
Fine, what next?
It is the direction of numbering of main chain. You can find that we can give numbering from either direction. So, we have to see the least sum of locants. Surprisingly it gives same number for sum of locants from both sides such as 3 + 4 =4 +3=7.
Then which direction is correct?
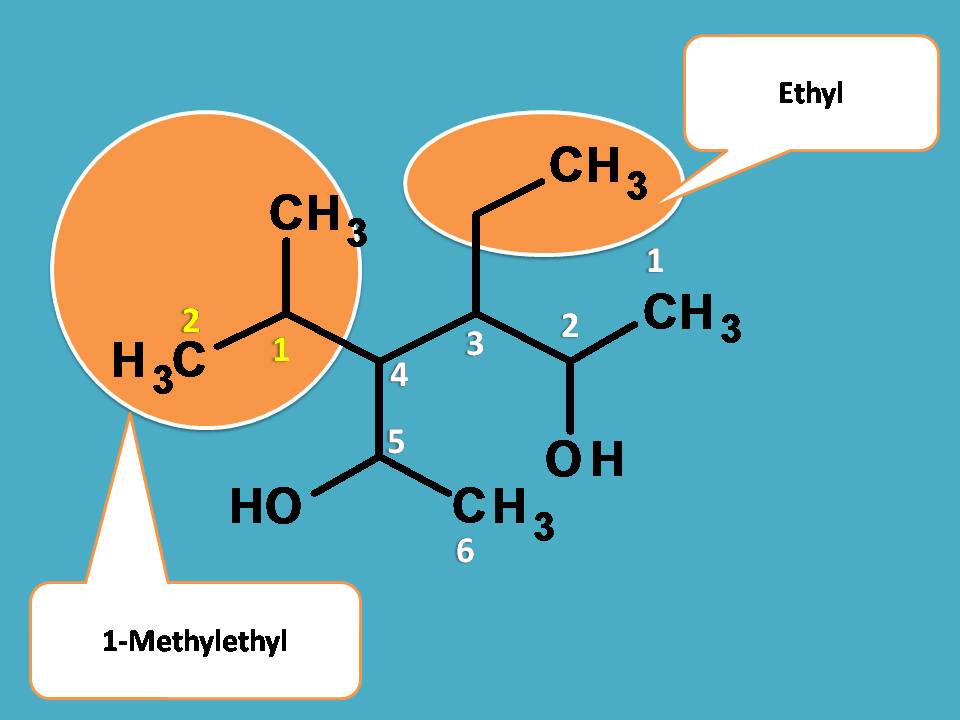
Here we have to use alphabetical order. The group that comes alphabetically first should be given least number. Here the side chains are one is ethyl and another one is methylethyl as we have seen in previous example.
Since ethyl starts with “e” whereas methylethyl starts with “m”, the former should be given least number.
Finally, hydroxyl groups are present at 2nd and 5th positions, hence suffix is 2,5-diol.
Therefore name of the compound is 3-Ethyl-4-(1-Methylethyl)hexane-2,5-diol.