Electronic transitions and types of electrons
by egpat 05 Jun 2019
What happens when a light falls on matter? Simply, molecule absorbs energy and the electrons are shifted from ground state to exited state by absorbing this energy. This electronic transition between the energy levels is the basis for both molecular and atomic absorption. Here we will discuss these type of transitions and how they can happen based on the electromagnetic radiation involved.
Types of electrons
Before going to electronic transitions directly, first of let’s discuss the types of electrons in an atom or molecule. Atom is a simple element with electrons distributed into the different shells. As it has no bonding, all the electrons are similar except in the fact that they have different energies according to the orbital in which they located.
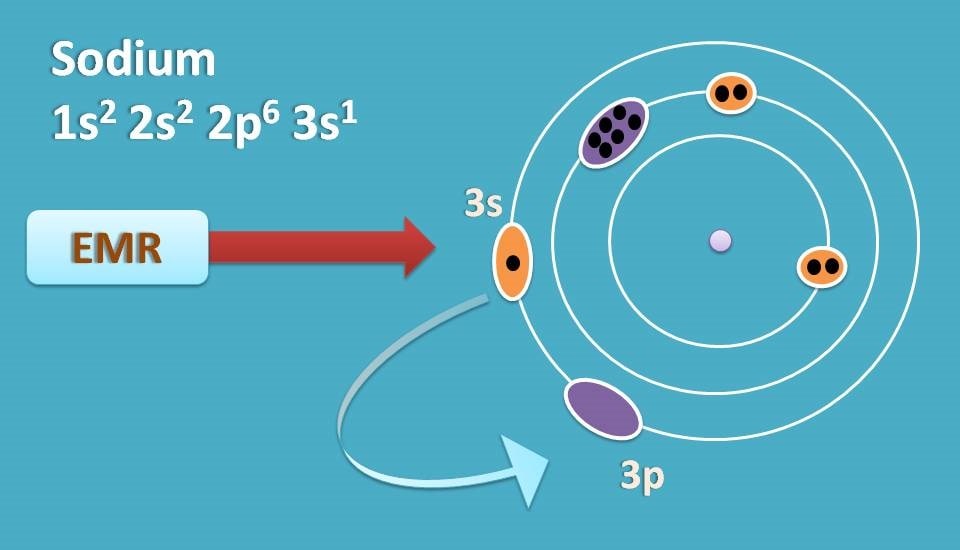
For instance, sodium has 10 inner electrons and one outer electron. Inner electrons are more stable and normally not interacted by UV-visible radiation. On the other hand, outer electrons are loosely held and they can easily undergo electronic transition when excited.
Now let’s turn to the case of molecules.
Molecules are not so simple as atoms as they have chemical bonding with other atoms. Therefore here we can list different types of electrons that may be present in the molecule.
- Inner shell electrons
- Sigma electrons
- Pi electrons
- Non bonded electrons
Inner shell electrons
Every atom in a molecule will have few electrons which are neither reside in outer orbital nor involved in bonding.
Let’s take water as example. Here oxygen is the central atom and it has electronic configuration as 1s2 2s2 2p4.
The electrons in 1s orbital are inner shell electrons and they are not involved in any bonding. These are highly stable and require high energy to produce a transition. UV-visible radiation is not sufficient to do this hence these electrons are inactive in this region.

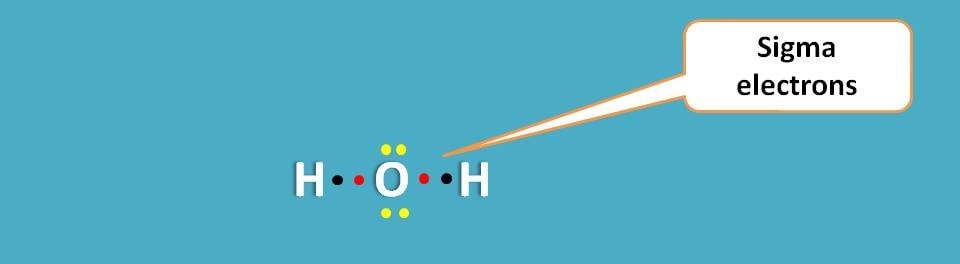
Sigma electrons
These are the electrons involved in sigma bonding between two atoms in the molecule. For example, in water molecule you can find oxygen to form two sigma bonds each with one hydrogen.
Pi electrons
These are the electrons involved in lateral ways of overlapping in the molecule forming pi bond. For instance, in acetaldehyde, carbon forms a pi bond with oxygen.
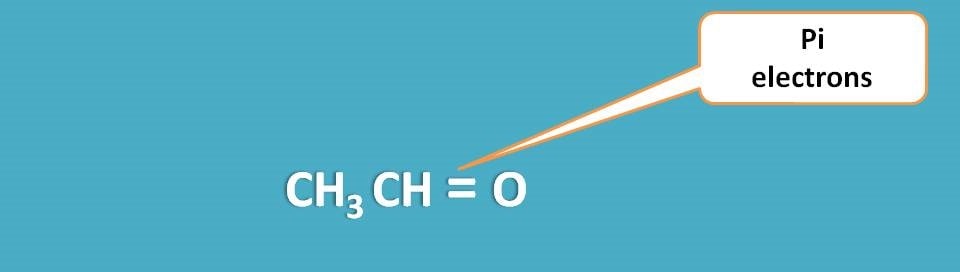
Again these electrons are stable but somewhat less than sigma electrons hence can be easily excited in UV-visible region.
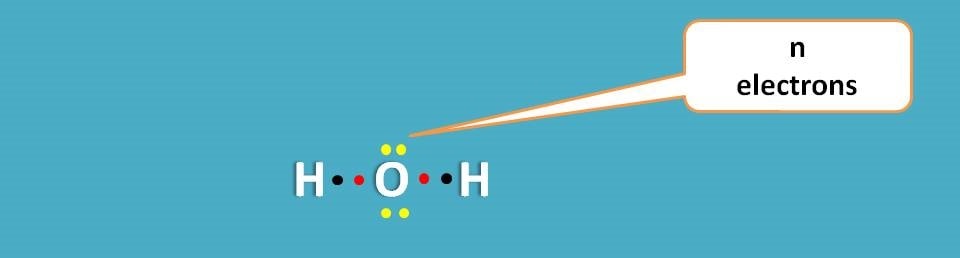
n electrons
These are the lone pair of electrons that are not involved in any bonding but still present in the outer shell. Hence these electrons can be easily excited.
Electronic transition in atoms
When light falls on the atom, the electrons in outer shell can go to excited state responsible for atomic absorption.
For example, sodium has electronic configuration 1s2 2s2 2p6 3s1. When it is excited in flame, one of the electron from 3s1 is shifted to the next orbit. In this process if absorbs energy.
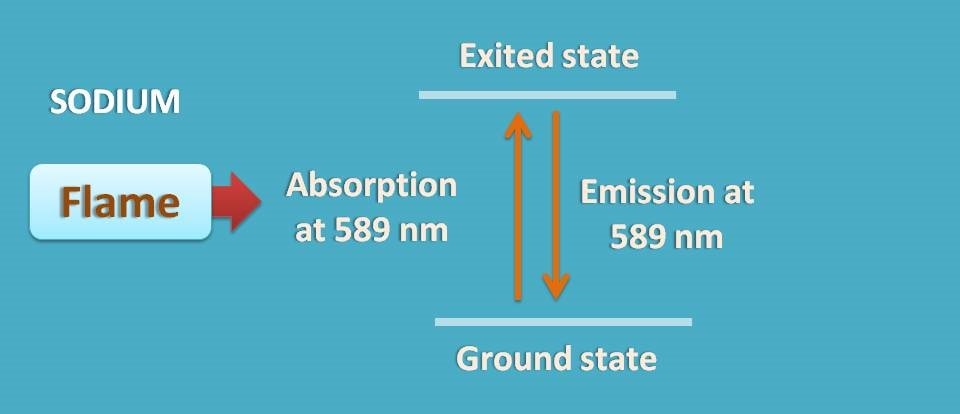
Now the electron is in a excited state which is not stable, therefore again jumps to 3s1 releasing energy. This is atomic emission.
Why sodium is golden yellow when burnt in flame?
Sodium when excited in flame electron jumps to excited level and then again jumps back to ground state releasing same amount of the energy as it absorbed. For sodium this falls at 589 nm which results in golden yellow colour in the flame.
Electronic transitions in molecules
As we have discussed above, in a molecule four types of electrons are involved among which only three types of electrons exist in outer shell. They are sigma, pi and n electrons.
We already know that stability of these electrons is as follows.
Sigma > Pi > n electrons
Among these sigma and pi electrons are located in bonding molecular orbitals when they form chemical bond in the molecule. These molecular orbitals just like atomic orbitals can exist in two states,
- HOMO
- LUMO
HOMO is the highest energy occupied molecular orbital that corresponds to ground state whereas LUMO is the Lowest energy unoccupied molecular orbital corresponds to excited state.
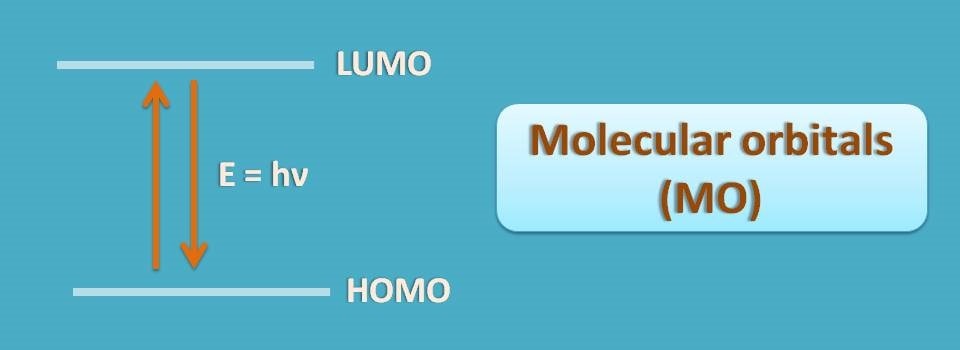
Therefore, an electron in HOMO can jump to LUMO when we supply energy in the form of electromagnetic radiation. This forms the basis for molecular absorption of EMR.
But here, unlike atoms, the situation is not so simple as different types of electrons present. Let us go in detail.
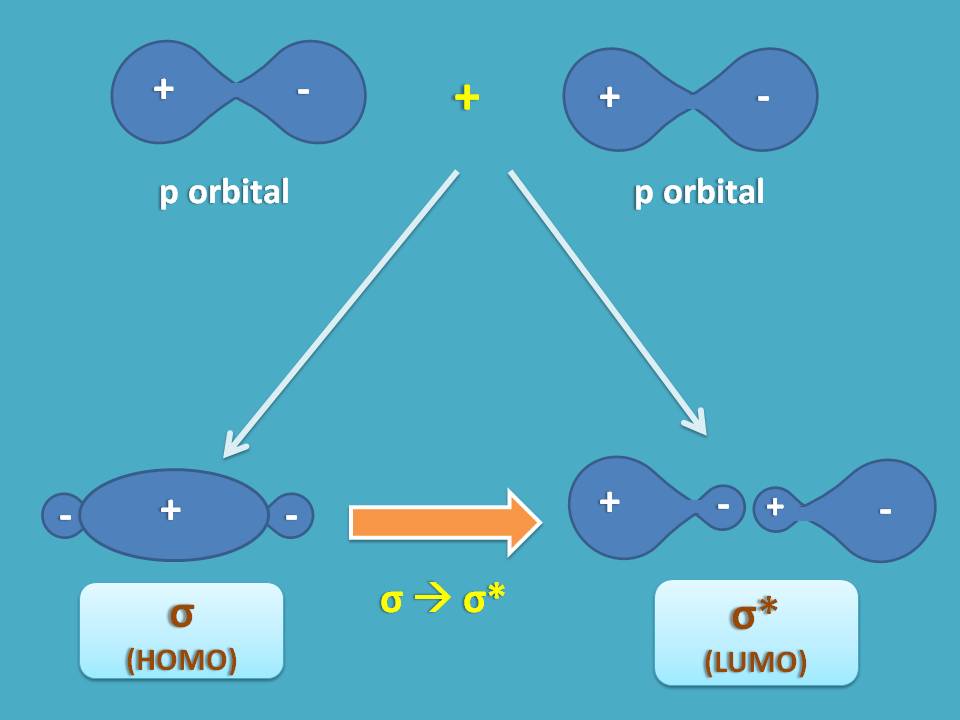
Suppose a sigma bond is formed between two p orbitals by sideways overlap. Two types of overlap may be possible. One is the bonding molecular orbital which is more stable acting as HOMO and another is the anti bonding molecular orbital acting as LUMO.
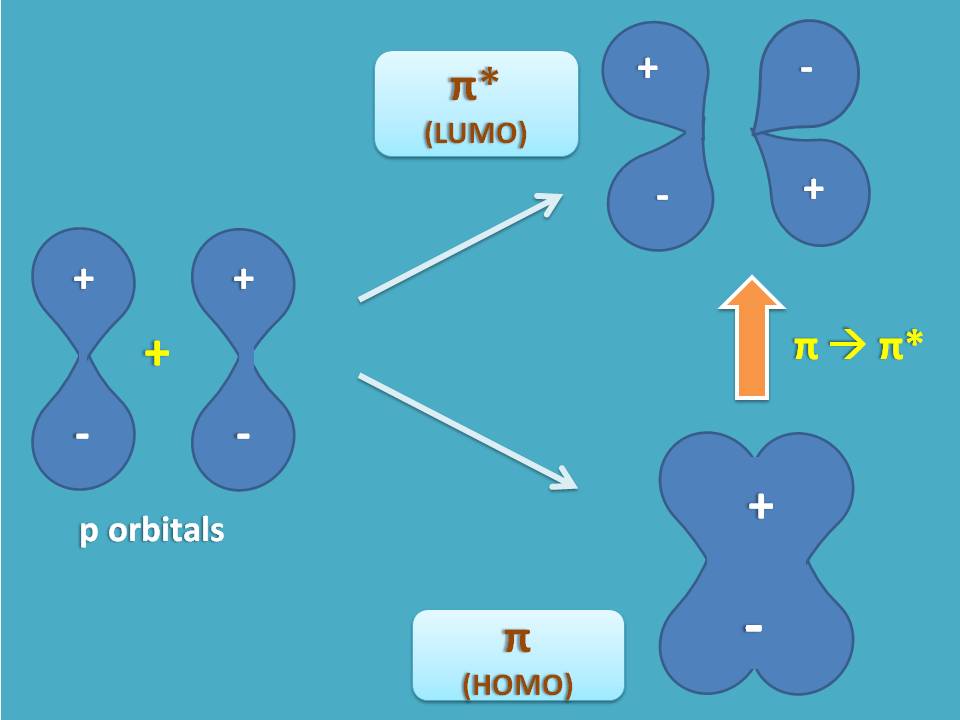
Similarly, when two p orbital overlap laterally to form pi bond, two types of molecular orbitals are formed one being bonding MO and another being antibonding MO.
On the other hand, lone pair of electrons are non bonding electrons that are not involved in any bonding hence can’t form any molecular orbital.
Now we can list out different types of electronic states in a molecule and corresponding transitions.
- 2 bonding molecular orbtials – σ and π
- 2 Anti-bonding molecular orbtials – σ* and π*
- 1 non-bonding electron – n
Arranging them in the energy order, σ bonding MO is least energetic and more stable while σ* anti-bonding MO is most energetic and less stable.
σ* > π* > n > π > σ
We can now draw an energy diagram and plot the possible electronic transitions here.
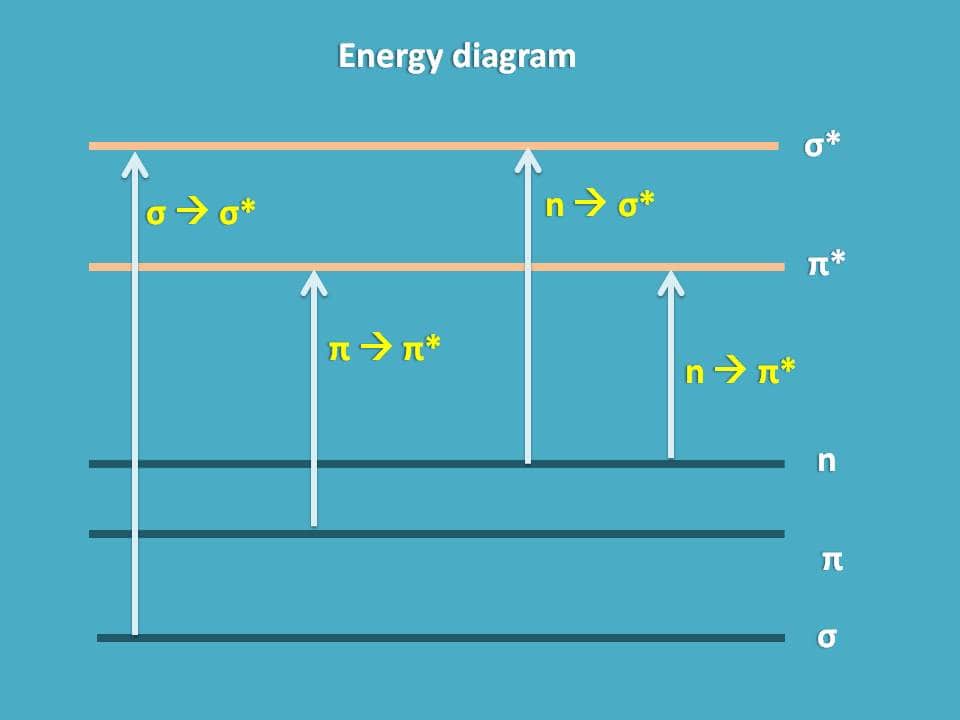
From the above energy diagram, you can easily identify which transition has more energy gap and requires more energy for the transition. So totally we have 4 possible electronic transitions with energy requirements for each transition in the following order.
σ → σ* > n → σ* > π → π* > π→ π*
Note: Transitions of σ → π* and π→ σ* are practically not allowed.
σ → σ* transition
You can find that energy gap between σ and σ* is very large and requires large amount of energy which can be supplied by vacuum UV radiation. So, all these types of transitions are possible around 10 nm to 200 nm.
Let’s take one example. Consider ethane molecule.
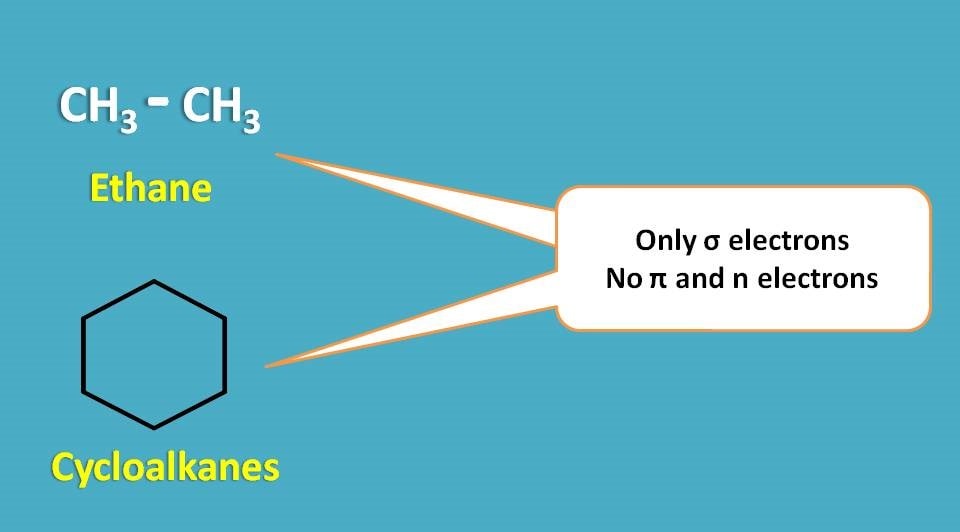
As it has only σ electrons, it can undergo only σ → σ* transition and absorbs only in vacuum UV region. Similarly alkanes and cyclcoalkanes can only show σ → σ* transition.
n→ σ*transition
Again this type of transition require high energy falling below vacuum UV region. But here the molecule should have both σ and n electrons to show this transition.
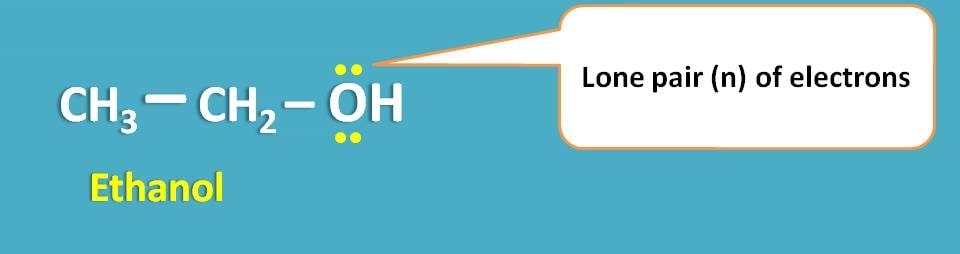
For example, ethanol has both σ and n electrons and therefore can undergo both σ → σ* and n → σ* transitions.

Why water is colorless?
As water has only σ and n electrons, it can undergo only σ → σ* and n → σ* transitions which fall in vacuum UV region. So in the visible region, water can’t absorb any radiation and hence colourless.
pi → pi * transition
One of the most important transitions that is responsible for colorful vision in p → p * transition. Any molecule with pi electrons can show such type of transition and the region of absorption may range from UV to visible radiation.
n → pi * Transition
Now this transition requires both n and p electrons. Consider acetaldehyde. It contains σ , p and n electrons hence it can undergo following types of transitions.

Among these n→p * transition requires least energy and falls under UV-visible region.
Surprisingly, even it requires less energy, the probability of this transition is somewhat less than p → p * transition
Electronic transition in inorganic metals
We know that potassium permanganate shows pink color. Who is responsible for its color as it doesn’t have σ , p and n electrons.

Here another type of transition comes into the play. With transition metals a new type of d → d* transition is possible responsible for the color.