Atomic spectra vs Molecular spectra
by egpat 05 Jun 2019
Sodium metal absorbs radiation at 589 nm and emits again at the same wavelength. In another words, it has similar absorption and emission spectra both involve a single wavelength resulting in line spectra. But in case of molecules the situation is different. Molecules show brand spectra and they absorb or emit not a specific wavelength but at a range of wavelengths. Here we will see why molecules differ from atoms in spectroscopy.
Line spectra by atoms
We know that atoms have definite atomic number and corresponding electronic configuration. That means, their electrons are distributed in fixed orbitals in each orbit.
For example, sodium has atomic number as 11 and its electronic configuration is 1s2 2s2 2p6 3s1. So its outer electrons are present in third orbit within s orbital.
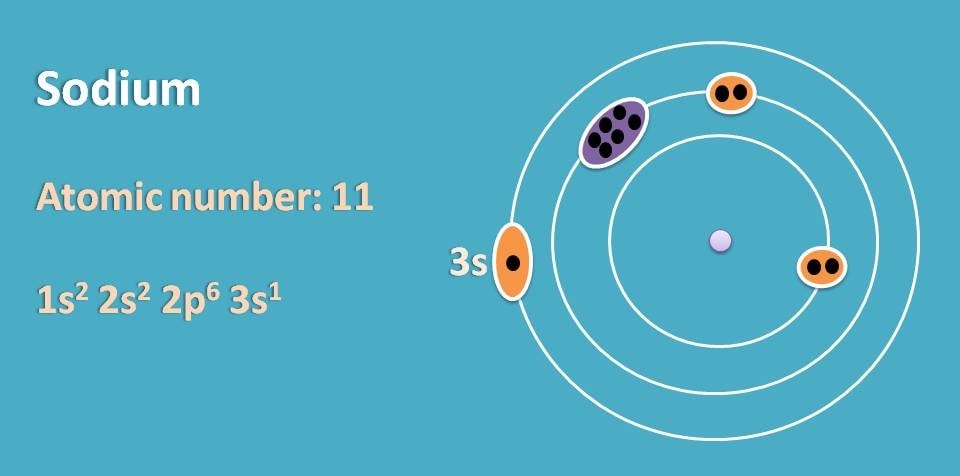
Similarly if we consider potassium, it has atomic number 19 and electronic configuration is 1s2 2s2 2p6 3s2 3p6 4s1. Here outer electrons are present in fourth orbit within s orbital.
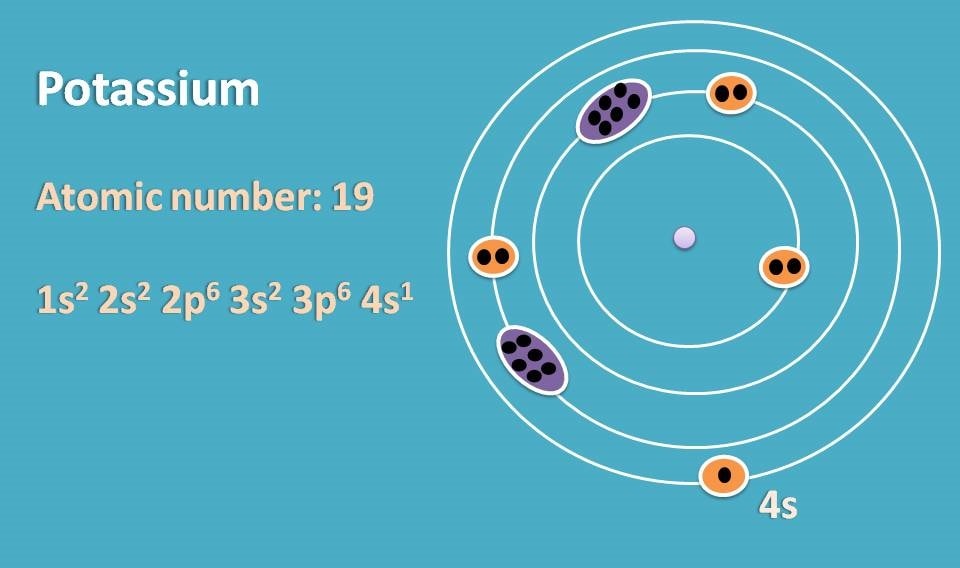
Now when we supply energy, the electron in the outer orbital can jump to the next orbital by absorbing the energy. Similarly, it can come back to original orbital by releasing the energy.
One of the interesting facts with atoms is that the energy associated with each orbit is fixed. So if an electron is third orbit, it will have a definite energy while fourth orbit another specified energy. By this way, the energy gap between any two orbitals is fixed.
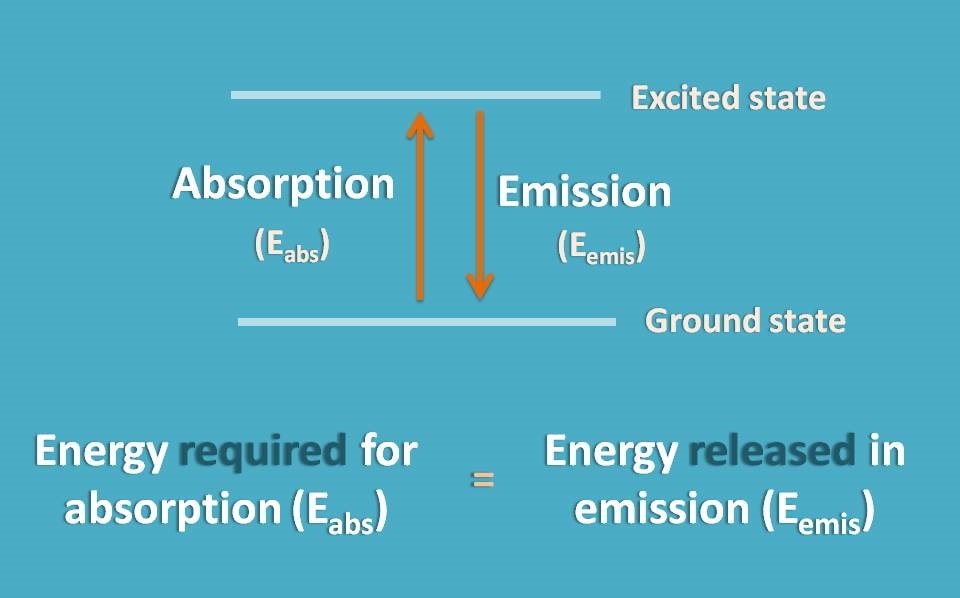
So whatever the energy required for an electron to jump from third orbit to fourth orbit is fixed and equal to the energy released by electron jumping back from fourth orbit to third orbit.
In this way, atomic spectra always produce line spectra at definite wavelengths.
Band spectra by molecules
Now let’s turn our discussion on molecules. The situation is not so simple, as molecules are made up of atoms with variety of linkages such as sigma bond and pi bond. Every chemical bond is like a spring that always produces some vibrations between the atoms in molecule.
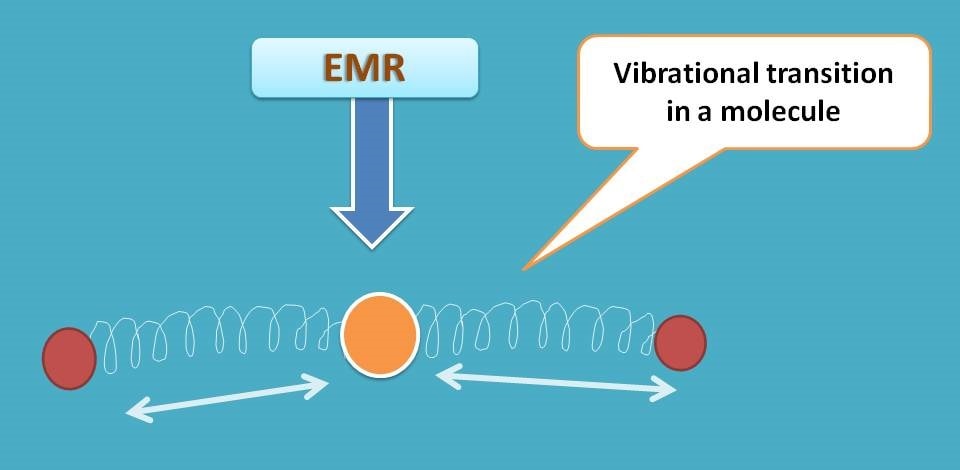
So when a light falls on a molecule, the energy is utilized for both electronic and vibrational transitions. Of course, the majority of the absorbed energy is utilized for electronic transitions and then for vibrational transitions. Electrons can also have rotational transitions which are not that much significant in molecular absorption and emission.
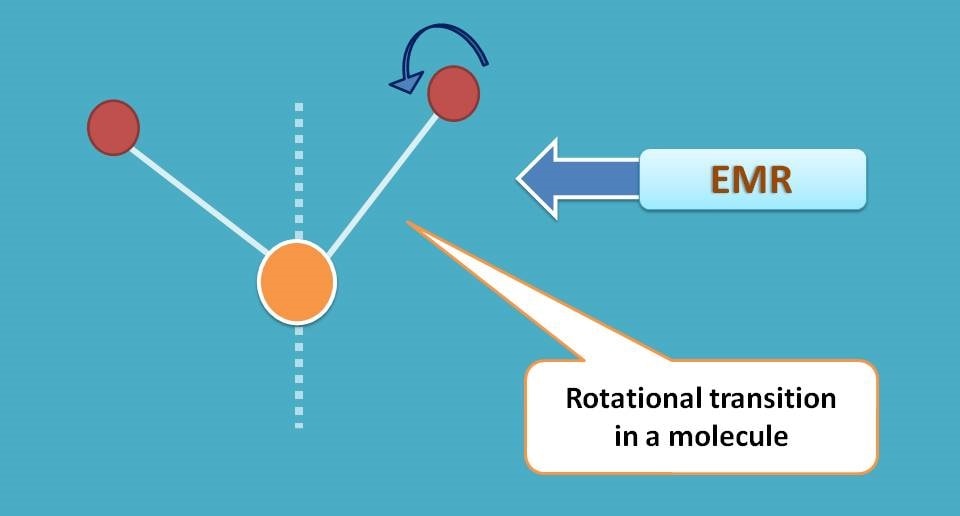
Now electrons in a molecule may have three in states at any moment.
- Electronic state
- Vibrational state
- Rotational state
Accordingly it will have corresponding energies associated with each state. Every electronic state is associated with several vibrational states and every vibrational state is associated with several rotational states.
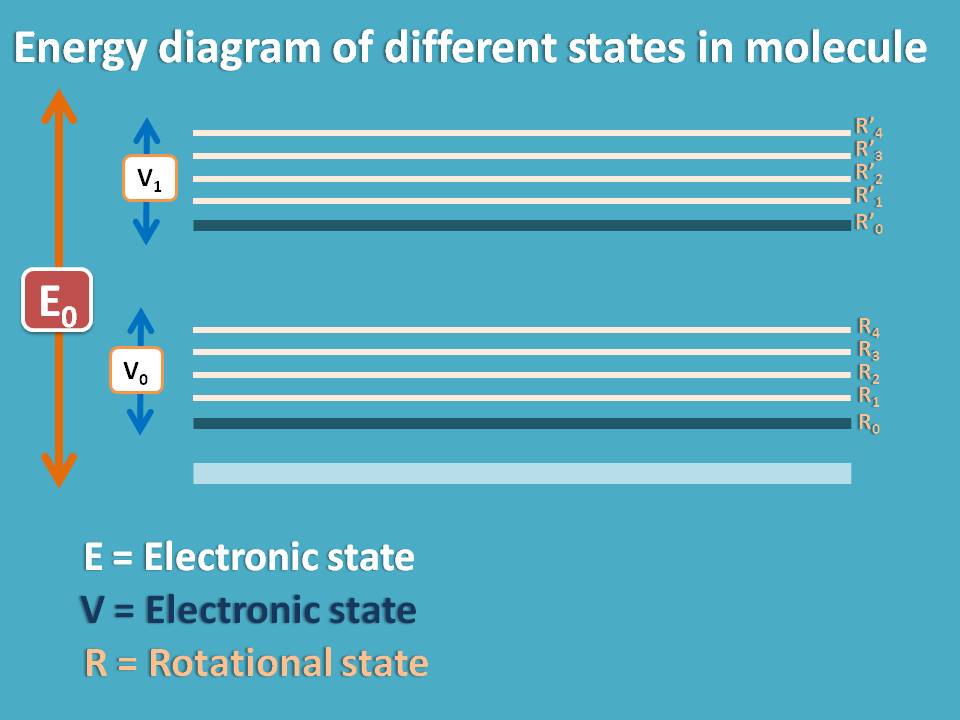
In order to understand the process very easily let’s take an illustration. Suppose, in an apartment you have traveled from ground floor to fifth floor by using lift. Now then you want to come down to the ground floor you may plenty of options. You may catch again the lift to go down to the ground floor or else if you are so energetic you can go the ground floor though the staircase.
You can also go to the fourth floor though staircase and then catch the lift to go down the ground floor. In this way, you can choose any option to come back to your starting point.
Similar is the case with electrons in a molecule. Suppose an electron is traveled from ground electronic state to excited electronic state by absorbing energy. Now it can come down by releasing the energy as vibrations else it can release part of energy as vibrations and remaining as radiation.
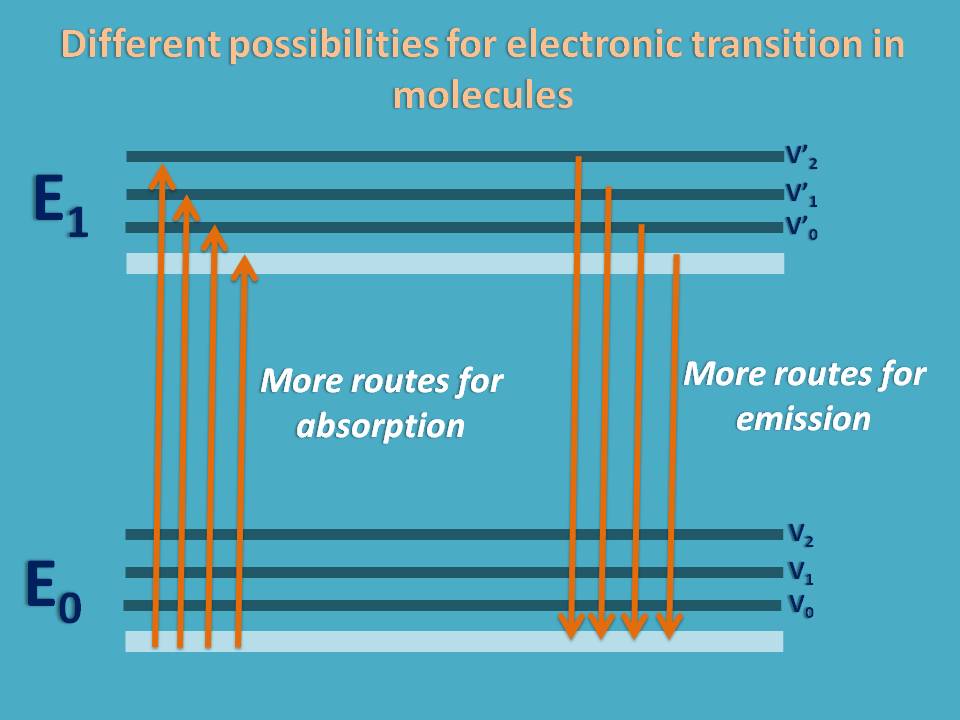
Due to several possibilities for emission, molecular spectra can’t occur at fixed wavelength but occurs at a range of wavelengths. But still molecule can show a wavelength at which it can either have maximum absorption or emission. This wavelength is generally designated as lambda max (λmax).