Aromaticity – 4 criteria every compound needs
by egpat 18 Mar 2019
We usually know that phenol is aromatic while alcohol is aliphatic. How could we say that? Even both have hydroxyl group still one is aromatic while other is not. What makes the difference?
Yes, it’s all due to those little double bonds that join their hands with single bonds on both sides, yet arranged systematically throughout the ring bringing a unique property to it....that is Aromaticity !!
Here we will go with all the prerequisites required for a compound to be aromatic along with examples. Let’s start.
1. Conjugation
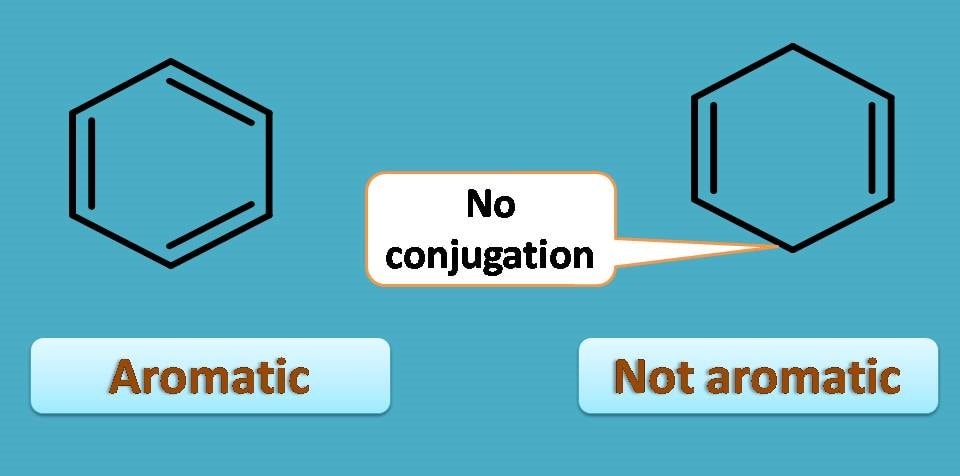
The unique pattern of alternate arrangement of double and single bonds is none other than conjugation that make the compound stand unique among the fellow organic compounds. For example, in the following compounds, the first structure has conjugated double bonds while second structure has no conjugation. Therefore later compound is not aromatic!
2. Cyclic structure
Just we have seen that conjugation is an important prerequisite for a compound to be aromatic. But is that property what we call conjugation, sufficient to win the game? Certainly, it’s not! Let’s take another example. Consider 1,3,5-hexatriene. Is it aromatic?
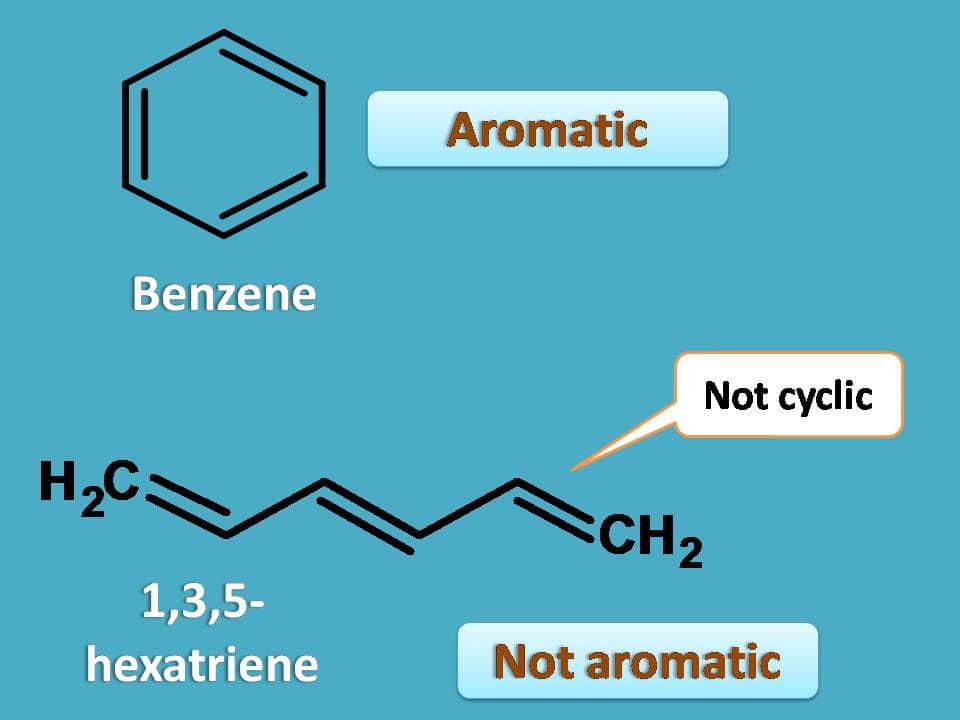
Undoubtedly it is not aromatic even it has conjugated double bonds. It can be easily visualised by comparing relative stability and chemical properties of these two compounds.
So conjugation is not only the factor responsible for aromaticity but still another factor plays important role. By this time, you can easily understand that another property required for aromaticity is the cyclic structure. 1,3,5-hexatriene is conjugated but it is not cyclic hence not aromatic while benzene is both cyclic and conjugated hence aromatic.
3. Number of pi electrons
It’s great that now we know two conditions that required for a compound to be aromatic. Is anything else?
Let’s take one example.
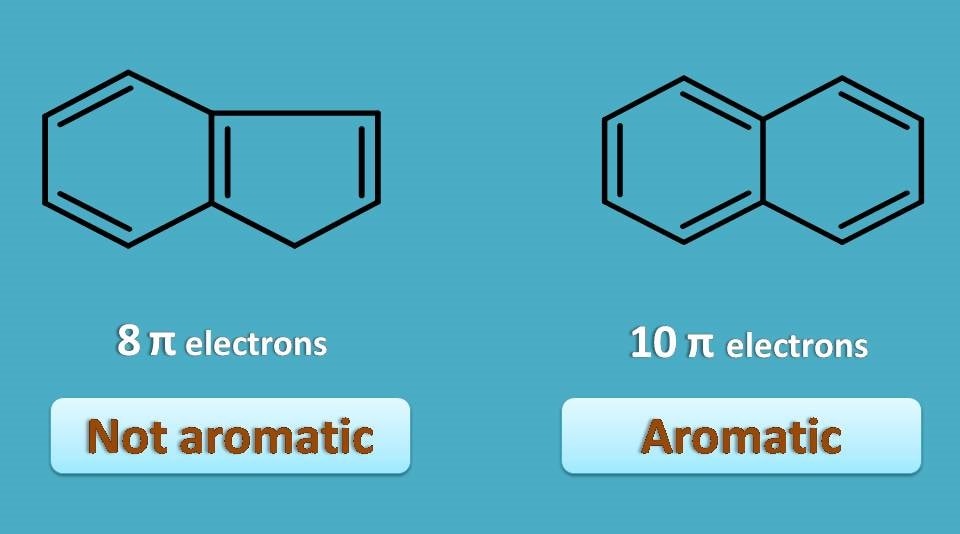
Here both the compounds are cyclic and conjugated still one is aromatic while other is not. The first structure contains totally six pi electrons and aromatic while the second structure has 8 pi electrons but not aromatic.
Is there any rule relating number of pi electrons in the ring and aromaticity?
Yes, of course, you may be already familiar with that rule, Huckel’s rule or 4n+2 pi rule. It simply states that,an aromatic ring should have 4n+2 pi electrons.
Let’s substitute different values for n such as n=0,1,2,3…..and so on.
If n=0, it will be 4(0)+2=2
If n=1, it will be 4(1)+2=6
If n=2, it will be 4(2)+2=10
In this way 2, 6,10,14,18…..and so on are the number of pi electrons that are only allowed for aromaticity. You can easily observe that all these numbers differ by 4.
So, in the previous example ring containing 6 pi electrons is only aromatic.
You can observe that all the numbers stated above are even. As each pi bond contains two pi electrons, its quiet natural that the number of pi electrons allowed in an aromatic compound is always an even number.
Stated in another way, the number of pi bonds allowed in an aromatic compound will be 1, 3,5,7 and so on.
Quite interestingly, all these are odd numbers!! So, we can state Huckel’s rule in another way.
“An aromatic ruing should have odd number of pi electrons”
4. Role of lone pairs
By the previous discussion, now we can clearly identify which is aromatic and which is not aromatic.
Let’s take one example.
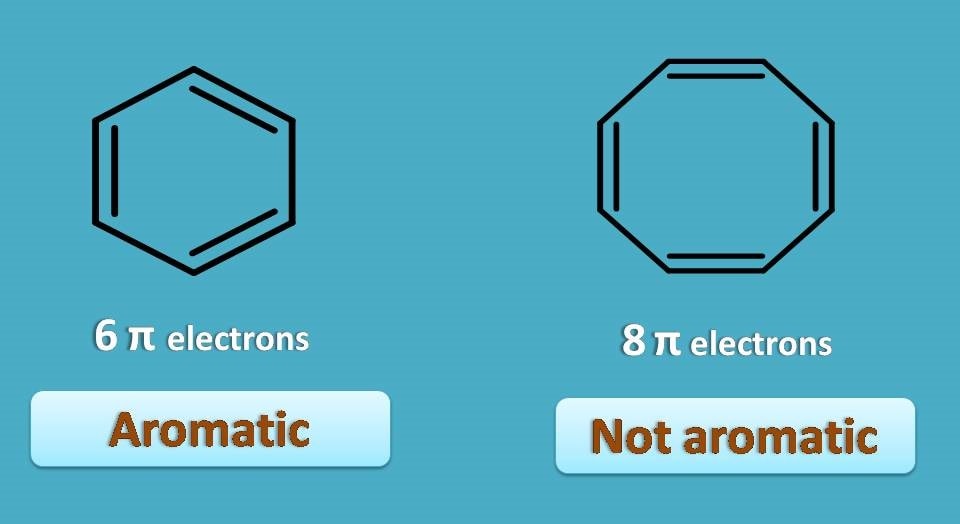
In the above example, you can easily find that first structure is not aromatic as it contains 8 pi electrons as well as it is not completely conjugated. On the other hand, the second structure is aromatic as it is cyclic, conjugated with 10 pi electrons or 5 pi bonds, all the requirements met for a compound to be aromatic.
Now let’s turn into another example, still little bit interesting.
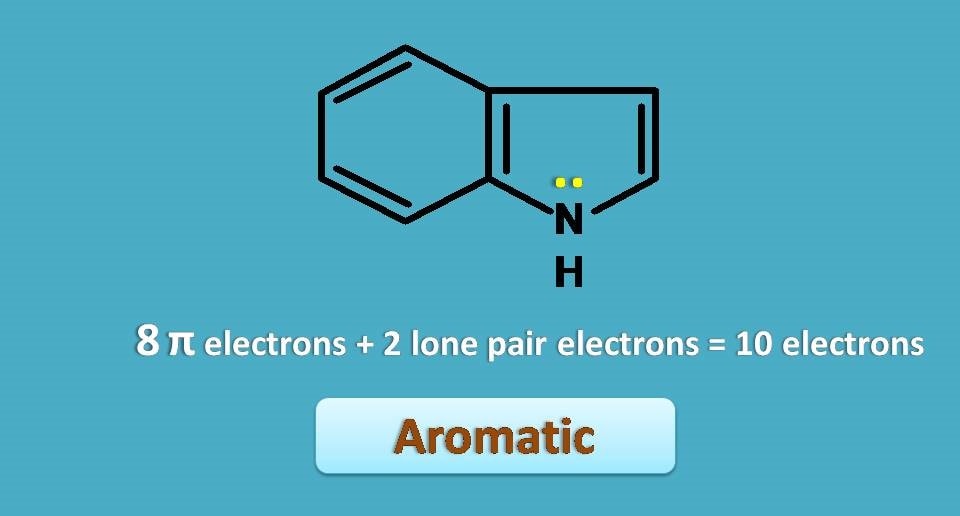
Is that compound aronmatic? By the first look and counting pi electrons you may declare that is not aromatic as it contains 8 pi electrons. But still the compound is aromatic!!
Here lone pair of electrons comes into the play. These electrons participate in the pi cloud resulting in stable uninterrupted pi cloud above and below the molecule making total number of electrons included in the pi cloud as again 10 hence aromatic.
It’s great, now let’s go with another example.
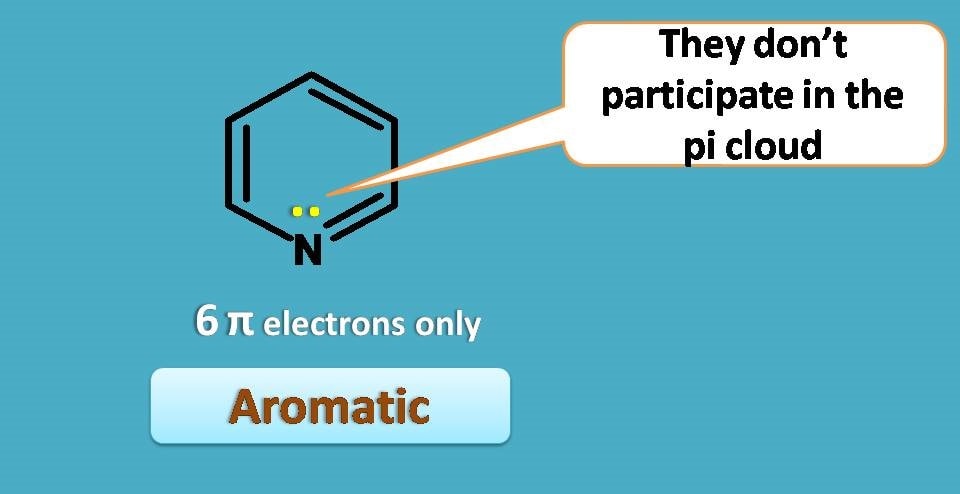
Is that aromatic? Now by using the same logic as above , total number of electrons that participate in the pi cloud is 6+2=8. So we may flag this compound as not aromatic. Surprisingly, this compound is also aromatic.
How it’s possible?
As the already attained 6 pi electrons through the pi bonds, there is no need of participation of lone pair of electrons in pi cloud, so they will be out of the ring.
In this way, lone pair of electrons play key role in stabilising the ring. Whenever deficiency is there they will supply the electrons otherwise not ultimately stabilising the ring towards aromaticity.
5. Planar structure
In an aromatic compound, a pi cloud is formed by pi electrons and sometimes lone pair of electrons all that bringing the total count as 4n+2.
But who forms this pi cloud? Undoubtedly, the p orbitals which are perpendicular the ring can form pi cloud. In another words, the ring should be planar where these p orbitals can form a stable pi cloud without a strain.
So, planarity or flat structure is another essential feature for aromaticity. To be more clear, let’s take one example.
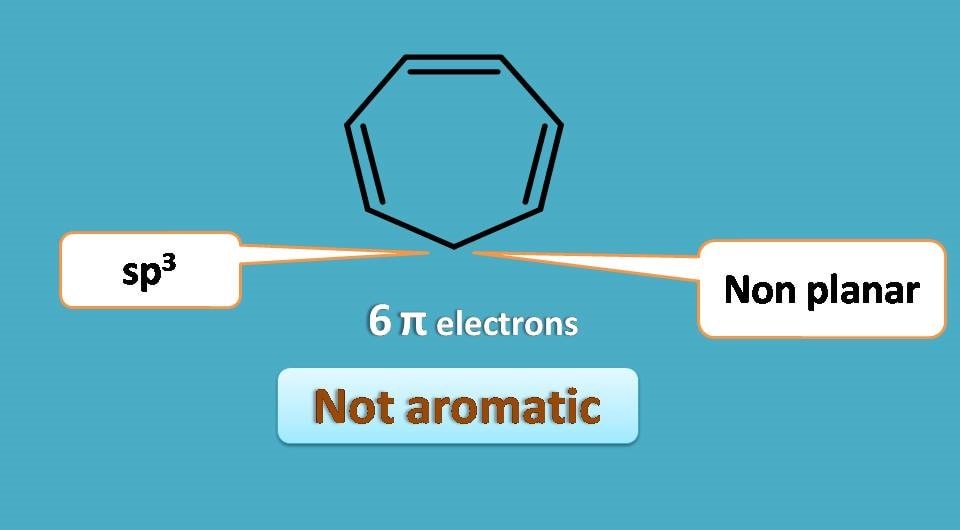
The above structure is cyclic and contains 6 pi electrons in somewhat conjugation. But conjugation was lost at one carbon which is saturated hence sp3 hybridised. So this carbon will not be oriented within the same plane as other carbons in the structure do, therefore the ring is not completely flat and the compound is not aromatic.
You can also observe that the structure has three conjugated double bonds but they are not continuous enough! In another words, the pi cloud is interrupted by a carbon bearing no double bond.
So we can define that an uninterrupted pi cloud is essential for aromaticity.
Conclusion
Aromaticity is a unique property shown by few of the cyclic compounds having pi electrons. For a compound to be aromatic, it should be cyclic, planar, contain uninterrupted pi cloud above and below the plane with 4n+2 pi electrons. One of the quite interesting fact is that the lone pair of electrons can also be involved in the pi cloud to make a stable aromatic ring.
Hope you enjoyed reading the article, post your comments if any in the below section. Please don’t forget to share this post with your friends. Have a great day!!