14 Rules to write chemical name by IUPAC nomenclature
by egpat 20 Mar 2019
Naming of compounds in organic chemistry is an important aspect in order to identify the specific structure. Structures can be named in different ways sometimes using by their common names and sometimes by using a nomenclature. Chemical name by IUPAC is a well accepted and official nomenclature for naming of organic compounds.
Where to start? Which rule should be applied first?
This is a big question, as few rules are required to follow in the fixed order while few of other rules can be used at anytime without any sequence while writing chemical name of compounds.
For example, you can’t select the longest chain or even start its numbering before you decide which the principal functional group in the compound is.
So, here we will discuss the 14 essential IUPAC rules required to write chemical name in organic chemistry. Let’s start with few of the basic rules that should be followed in the same order as discussed here.
Rule 1: Identification of principal functional group.
First step in writing chemical name for a given structure b IUPAC is to identify the principal functional group in the structure. If the structure contains only one functional group, it can be directly considered as the principal functional group.
For example,
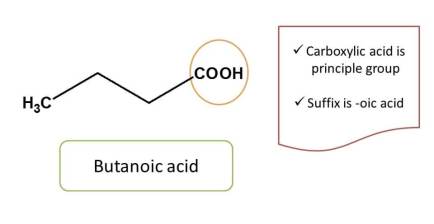
But in many cases, compounds will have more than one functional group. In such situations, the principal functional group is determined by the priority order.
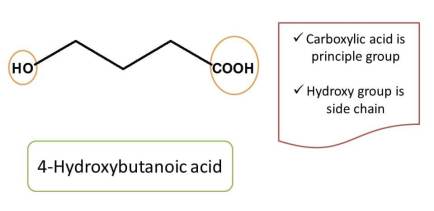
The highest priority group is considered as principal functional group and remaining all other functional groups are treated as side chains.
To learn how to determine the priority, see IUPAC priority order of functional groups.
If we consider the above example, carboxylic acid should be given more preference than hydroxyl group according to IUPAC nomenclature for organic compounds. Hence chemical name of the compound is 4-hydroxybutanoic acid.
Rule 2 : Selection of parent chain
Second task in organic chemistry naming is to select the parent chain. Parent chain can be selected as the longest chain including principal functional group.
The longest possible chain with principal functional group is treated as parent chain.
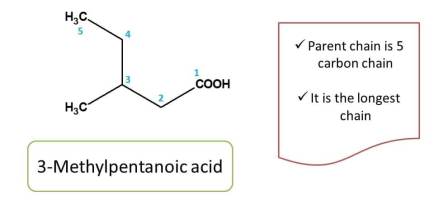
For example,
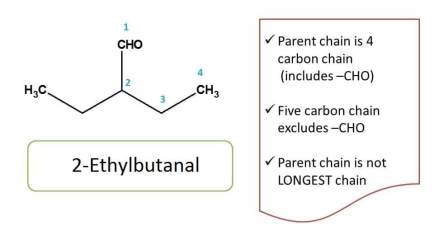
Here straight chain is the longest chain with five carbons, but it should not be selected as parent chain as it does not include principal functional group (-CHO). So we have to select a chain that should include principal functional group. Hence chemical name of the compound is 2-ethylbutanal.
Rule 3 : Selection of parent chain from more than one possibility
As we have just seen above, a parent chain should be longest chain including principal functional group.
But in few cases of organic chemistry naming, we can observe more than one chain meeting the above criteria. Let’s see the following example.
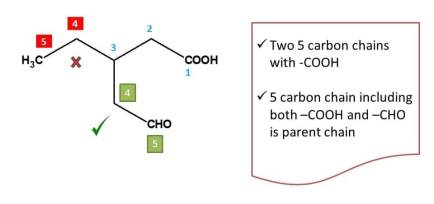
It has two longest chains both including the principal functional group. In such situations, we can decide the parent chain by testing the following criteria one after another.
Criteria 1 : Chain containing maximum number of functional groups
Criteria 2 : Chain containing maximum number of side chains
Let's see an example for first criteria.
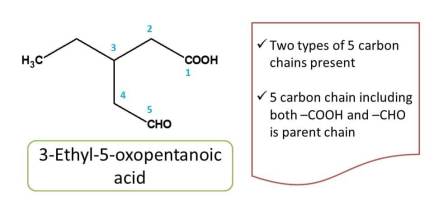
In the above example, two longest chains are possible. So, we have two apply first criteria i.e. chain containing maximum number of functional groups. The chain with numbering indicated by green color contains two functional groups viz. -COOH and -CHO whereas other chain indicated by red color numbering includes only one functional group (-COOH). Hence the chemical name of the compound is 3-ethy-5-oxopentanoic acid.
Now let's take another example using second criteria.
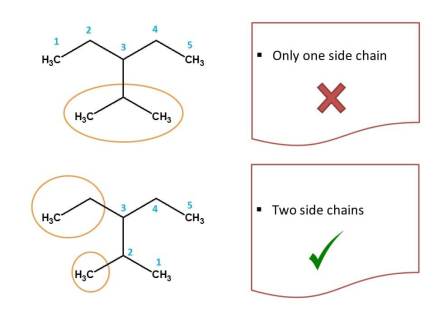
In the above structure, two types of longest chains possible each with 5 carbons. So let's apply first criteria. Again two types of chains are possible both including functional group(-CHO). Hence we should check second criteria i.e. chain with maximum number of side chains. As shown above, first method of numbering contains only one side chain, whereas second method contains two side chains and therefore it is correct.
Rule 4 : Give the root name
Root name of the compound can be given by counting the number of carbons in the parent chain. According to IUPAC nomenclature for organic compounds, root names can be given as following.
| No. of carbons | root name | No. of carbons | root name | |
| 1 | meth | 6 | hexane | |
| 2 | eth | 7 | hept | |
| 3 | prop | 8 | oct | |
| 4 | but | 9 | non | |
| 5 | pent | 10 | dec |
Example :
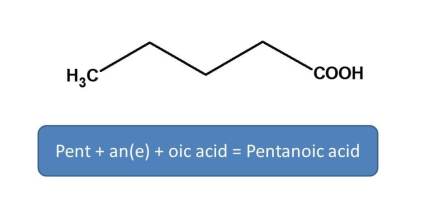
Here principal functional group is carboxylic acid, hence suffix is "-oic acid". So we have to replace "e" in ane with "oic acid". Here five carbons,therefore root name is “Pent-”. Now combining all,
Pent + an(e) + oic acid=Pentatonic acid
Rule 5 : Give the numbering
Now we have selected principal functional group, parent chain and root name. Next step in IUPAC nomenclature is to give numbering to the compound in order to identify the location of the side chains.
Numbering should be done from that direction which gives least number to the principal functional group.
Let's take simple example.
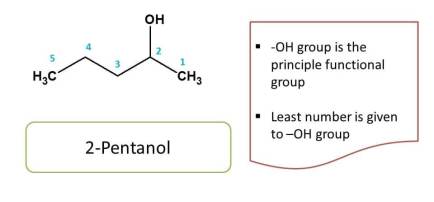
Here the principal functional group is hydroxyl group (-OH), hence suffix is "-ol". Now numbering can be done from either direction. Numbering from right to left gives 2 and left to right gives 4 as locant to hydroxyl group. As we have to provide least locant possible to principal functional group, the first direction is correct. Hence chemical name of the compound is 2-pentanol.
Rule 6 : Numbering in case of more than one possibility
In case of two or more possibilities, the numbering should be done according to the following criteria one by one.
- Criteria 1: Sum of the locants is least. (Least sum rule)
- Criteria 2:Least number is given to the side chain comes first alphabetically
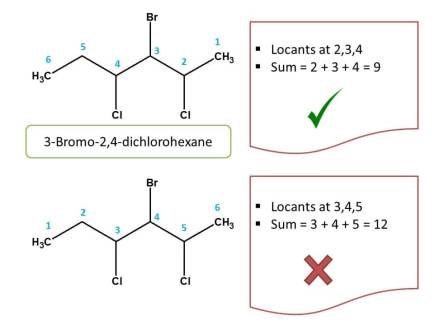
Again in the above structure, numbering can be done from either direction.
- Right to left: Locants chlorine and bromine are present at 2,3 and 4. Hence the sum of the locants is=2 + 3 + 4=9
- left to right: Here locants are present at 3,4 and 5. Hence the sum of the locants is=3 + 4 + 5=12
Since the first direction yields lowest sum of locants, that direction is correct. Therefore chemical name of the compound is 3-Bromo-2,4-dichlorohexane
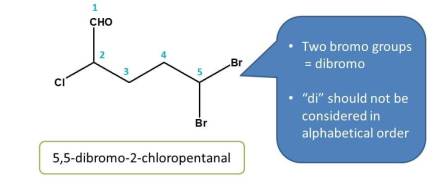
Criteria 2 - Side chains with alphabetical order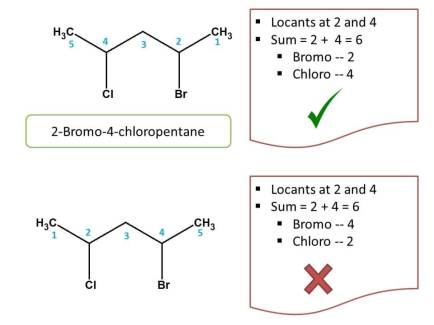
Now in the above example, we can clearly observe that two possibilities are there for numbering. So let's check criteria 1. Again sum of the locants from both the directions is same i.e. 2 + 4=6. So now we have to check next criteria i.e. alphabetical order.
Since bromine comes alphabetically first than chlorine least number should be given to bromine
- Right to left: Bromo was given location - 2
- left to right: Bromo was given location - 3
Obviously, the first direction is correct, hence chemical name of the compound is 2-Bromo-4-chloropentane
Other rules
Till now we have discussed basic rules required for IUPAC nomenclature of organic compounds that should be followed in a fixed order. Now we will see few of the rules which may be required in organic chemistry naming in other situations.
Rule 7 : The substitution on heteroatom
Atoms other than hydrogen and carbon are considered as heteroatoms. Common heteroatoms we observe in many of compounds include N,O,S and P etc. When these atoms are substituted, it should be indicated by corresponding prefix.
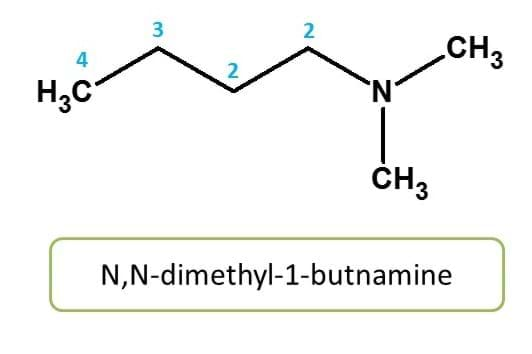
Here nitrogen is substituted by two methyl groups. Hence it should be indicated as N,N-dimethyl
Rule 8 : Numbering of the side chain
Numbering of the side chain should be started from the point of attachment even it bears any functional group. If the side chain is branched it is again numbered from the carbon which is attached directly to the parent chain.
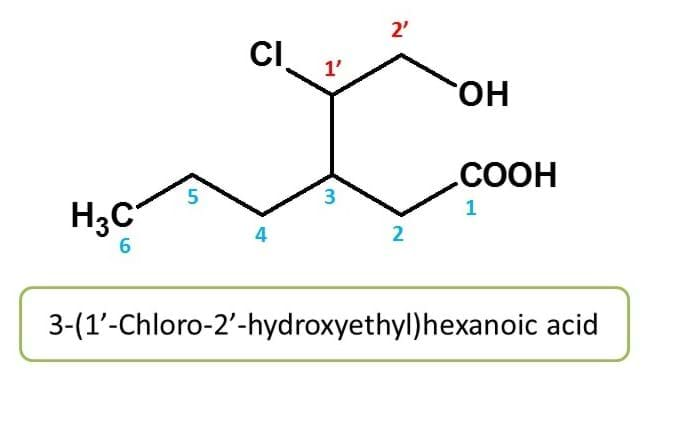
Here side chain numbering is given from point of attachment. It is a two carbon containing chain with chlorine at 1st position and hydroxyl group at 2nd position. Side chain numbering can be differentiated by using "prime" ( like 1' and 2'), but not essential. The entire side chain is attached to the main chain at 3rd position. Hence chemical name of the compound is 3-(1'-Chloro-2'-hydroyethyl)hexanoic acid.
Rule 9 : Multiple substituents
Substituents or side chains when present more than once on parent chain are represented by prefixes like di, tri, tetra and penta etc.
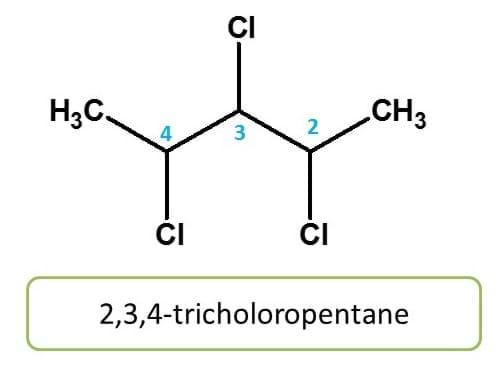
Here chlorine group is present three times at 2,3 and 4 locations. Therefore chemical name of the compound is 2,3,4-trichloropentane.
Rule 10 : Side chains with side chains
Sometimes we can find two or more side chains each having further side chains attached in similar way. In such cases these side chains are indicated by terms like bis-, tris-, tetrakis- and pentakis-based on two, three, four and five times they present.
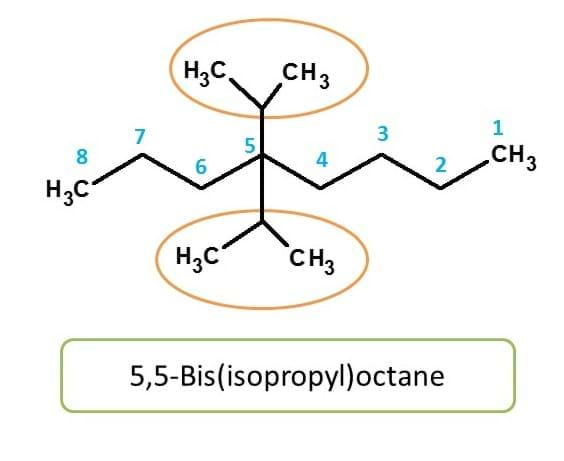
Here again, isopropyl group is attached to main chain in similar way, hence indicated by prefix like "bis".
Rule 11: Multiple groups joined by single bond
Generally two identical cyclic groups are joined through a carbon are just indicated as above by the term “di”.
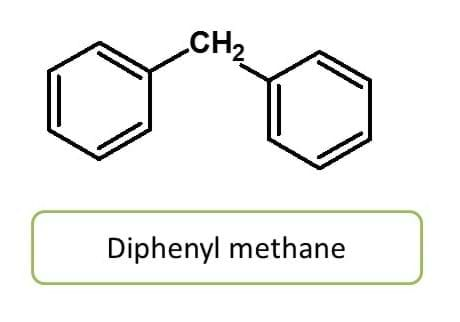
But few identical ring systems may be joined directly by single bond without any carbon between the two rings. These groups can be indicated by terms like bi-, ter- and quarter- etc.
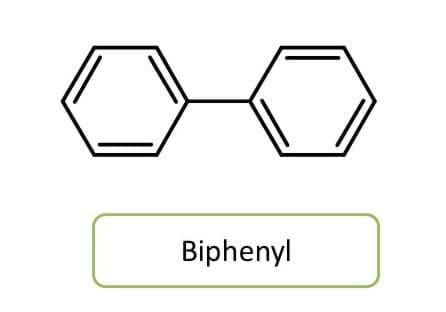
Note here that even the chemical name of the compound is ended with “yl”, it is not a radical name but it is a compound name.
Rule 12 : Arrangement in alphabetical order
All the side chains are arranged alphabetically prefixed by their positions on parent chain.
Rule 13 : Alphabetical order – includes and excludes
Prefixes are important as they give information of how the groups are connected to parent chain. While arranging in the alphabetical order by using IUPAC nomenclature for organic compounds, few prefixes are considered and few are excluded.
Prefixes excluded for alphabetical order:
- bi, ter and tetra
- di, tri, tetra
- sec, tert
Prefixes included for alphabetical order:
- Cyclo, benzo etc.
- 1H, 2H etc
- Iso, neo etc
Rule 14 : Naming of Radicals
Radicals are the side chains obtained from the removal of hydrogen from the corresponding hydrocarbon. IUPAC nomenclature for organic compounds considers these radicals as side chains which are indicated by their location on the parent chain.
Radicals can be denoted differently while providing organic chemistry naming based on the number of hydrogens removed from hydrocarbon. They can be univalent, divalent or trivalent, if number of carbons removed is one, two or three respectively.
Chemical names of these radicals can be obtained by replacing the suffix “ane” by the following suffix from the corresponding hydrocarbon.
| Radical | Suffix |
| Univalent | -yl |
| Bivalent | -ylidene |
| Trivalent | -yldyne |
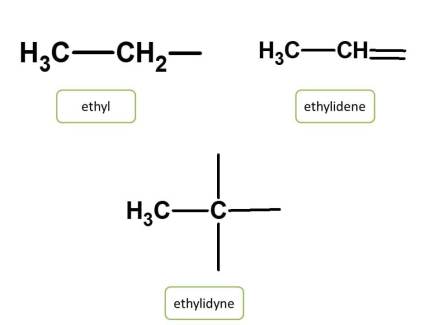
Let's see various examples for all these radicals.
Example 1 : -yl
Here side chain with two carbons is attached by single bond to parent chain. Hence it is indicated by "ethyl"
Example 2: -ylidene
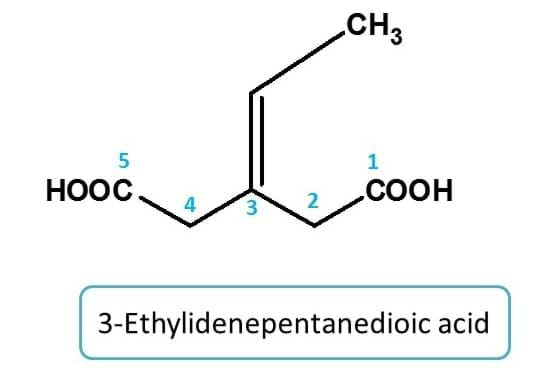
Here side chain with two carbons is attached by double bond to parent chain. Hence it is indicated by "ethylidene"
Example 3: -ylidyne
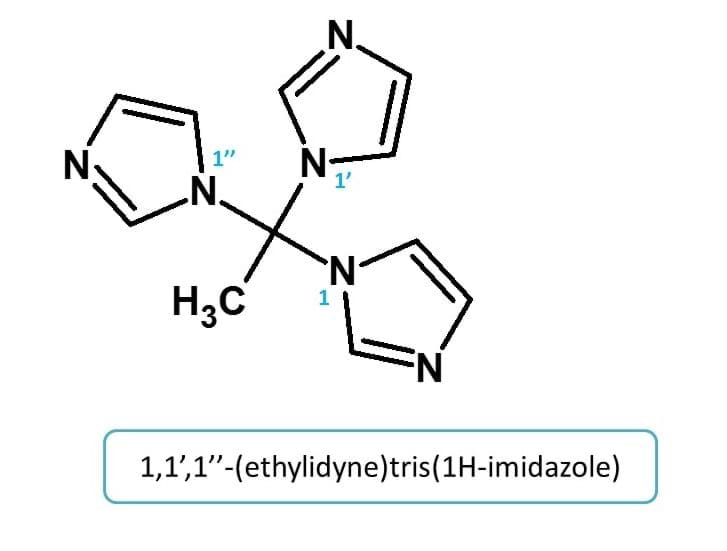
Here side chain with two carbons is attached to three identical imidazole rings. Hence it is indicated by "ethylidyne"
So, by using various rules in IUPAC nomenclature you can easily provide organic chemistry naming for many compounds without any ambiguity. The main aspect in the task is to proper use of IUPAC rules by considering all the possibilities and applying the right IUPAC rule for correct naming of organic compounds.